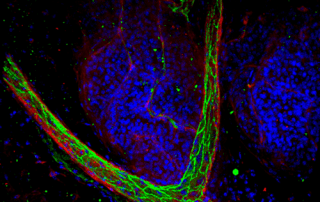
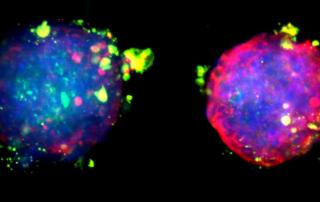
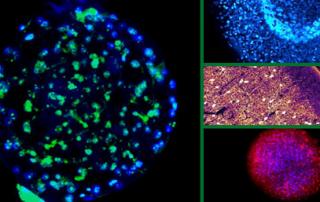
Though tissue clearing allows for an unprecedented view of tissues and the investigation of complex biological phenomenon, many researchers have struggled with adopting these techniques and successfully imaging their tissues in 3D. One of the main reasons for this adoption hurdle is that the 3D microscopic imaging of tissues requires knowledge in the fields of tissue labeling, tissue clearing, advanced microscopy and data processing.
Over the last two years Visikol has worked with hundreds of researchers to better understand the challenges in adopting tissues clearing and through this process have developed the easy-to-use Visikol® HISTO™ tissue clearing technique as well as the Visikol HISTO Protocol Guidebook. However, before a researcher attempts to adopt Visikol® HISTO™ tissue clearing it is important that they read through the below considerations as there are numerous myths and misconceptions surrounding the practice of tissue clearing.
1. Do not jump to trying to clear and label your whole tissue such as a mouse brain right away:
Start small with thin pieces and work your way up sequentially as you optimize for your label as it will save you significant time and money. A 1 mm brain section can take 15 hours to process with Visikol® HISTO™ for immunofluorescent labeling where a whole brain would take 14 days.
2. Determine what your imaging modality is capable of:
- Inverted Confocal Microscope – Typically can only image tissues to a depth of 2 mm due to refractive index mismatch. Inverted dipping objectives (e.g. ) can improve inverted confocal imaging depth but are rare and typically setup for high content imaging.
- Upright Confocal Microscope – Can image tissues to a depth of 2 mm with traditional air matched objectives. Deep tissue imaging requires dipping objectives that may not be compatible with solvents like Visikol® HISTO™ – please consult your imaging core manager about compatibility. If your objective is not compatible, you can use our ClearWell imaging chambers.
- Light Sheet Microscope – Designed for rapid whole tissue imaging at generally lower resolutions than confocal microscopy. Might not be directly compatible with solvent based clearing techniques like Visikol® HISTO™ and special specimen prep might be required – consult your imaging core manager or the Visikol team at info@visikol.com for guidance.
- Fluorescent Microscope – These microscopes do not have the ability to optically section tissues and thus are not a 3D microscopy modality. However, for higher throughput applications (e.g. 3D cell cultures), Visikol® HISTO-M™ can be used to dramatically increase the number of cells within models that are characterized.
- Photon Microscope – Have very variable performance depending upon their design – please consult your imaging core manager about your system’s functionality.
3. Understand the compatibly of your tissue clearing technique with materials.
Do not use polystyrene containers with Visikol® HISTO-2™ or other techniques such as BABB or 3DISCO as they will be degraded. Visikol® HISTO-M™ has been specifically designed to be compatible with polystyrene well plates.
4. After clearing do not switch your tissues from an aqueous mounting media
to a solvent based media or vice-versa as it will cause tissues to unclear. For Visikol® HISTO-1™, Visikol® HISTO-2™ and Visikol® HISTO-M™ do not mount your tissue in VECTASHIELD or any other media after clearing.
5. For imaging, tissues can be mounted in well plates if compatible with them (Visikol® HISTO-M™)
or tissues can be mounted in ClearWells. During imaging samples can be kept in place for long imaging runs using blue sticky tak.
6. If using fluorescent protein make sure not to use a methanol gradient
for dehydrating tissues as it will quench FP. Use ethanol at 4C instead.
7. If your tissue is not completely transparent don’t worry!
This just means that the tissue was not dehydrated or cleared in enough volume of solution or for long enough. Water being left behind causes the tissue to stay opaque. To fix this, just rehydrate and reclear the tissue using a larger volume and for a longer period of time. You can then optimize for time and volume with your specific type of tissue later.
8. Visikol® HISTO-2™ is hygroscopic
and thus it is very important to keep tissues sealed when not imaging. Water accumulating will cause clouding.
9. After labeling and clearing, tissues
can be stored for months prior to imaging so long as the container is sealed and is covered from light.
If you have any questions please contact Visikol at info@visikol.com