Visikol announces introduction of assay for screening islet cell proliferation using InSphero microtissues and advanced 3Screen™ imaging and analysis.
Understanding pancreatic β-cell function in the regulation of glucose homeostasis and metabolism is of paramount importance in the research of the most common human metabolic disease – diabetes. Although isolated primary islets are considered the gold standard tool of diabetes research, their experimental use is limited due to their inherent heterogeneity in size, cellular composition and purity, as well as their rapid decline in functionality and viability ex vivo.
The ability of the small molecule harmine to activate β-cell proliferation in vivo is a well-established phenomenon and, for this reason, has initiated interest developing analog compounds to treat diabetes. This in turn has given rise to interest in the development of high-throughput screening (HTS) assays to survey the efficacy of these drugs in in vitro models of pancreatic islets.
In this study, we combine the 3D InSight™ Human Islet Microtissues provided by InSphero and the Visikol HISTO-M tissue clearing reagent to illustrate the power of tissue clearing on high content screening (HCS) of novel drugs targeting β-cell proliferation.
Materials and Methods
Reagents and cell culture
3D InSight™ Human islet microtissues were obtained from InSphero Inc. Antibodies and fluorescent dyes and antibodies were obtained from Invitrogen (Thermo Scientific). Harmine was obtained from Sigma Aldrich.
Treatment of microtissues with antiproliferative cisplatin
3D InSight™ Islet Microtissues were prepared from human islets (Age 29, Male, Hispanic, BMI 22.17, HbA1c 5.5%) and delivered in an Akura™ 96 well plate. Medium exchanges were performed every 2 days to maintain the microtissues in culture. On day 19, a 4.5-day treatment with 10 µM Harmine was started, with re-dosing after 2.5 days. Harmine was dissolved in DMSO to make a 10 mM stock solution, and a vehicle control was treated alongside for comparison.
Fixation and immunolabeling
On day 23, microtissues were fixed using 4% PFA, followed by washing in PBS to remove fixative. Microtissues were treated with methanol, followed by 20% DMSO/methanol, followed by 0.2% Triton X-100 in PBS to improve penetration of antibodies. Microtissues were blocked with 10% donkey serum, then incubated with guinea pig anti-PDX1 (1:150 dilution, Abcam Cat# ab47308), rabbit anti-Ki67 antibody (1:200 dilution, Abcam Cat# ab15580), and with goat anti-rabbit AlexaFluor568 and goat anti-guinea pig AlexaFluor488 conjugated secondaries (1:250 dilution, available from Invitrogen) to label β-cells and proliferating cells. Nuclei were counterstained with DAPI (Molecular Probes).
Clearing and high throughput imaging of microtissues
Microtissues were dehydrated with methanol, then cleared with Visikol HISTO-M. Imaging of spheroids was conducted in InSphero Akura™ 96 well plates using a CX7-LZR High Content Screening platform confocal imager (ThermoFisher). Multi-channel z-stacks were collected for each tissue, using 5 µm steps at 20X magnification. Images were processed using automated ImageJ macros, and cells were counted using CellProfiler.
Results and Discussion
Drug-induced β-Cell Proliferation
The islet microtissues were treated with harmine at a concentration known to induce significant proliferation in dispersed islets cells (10 µM). After intervention with harmine, the effects on islet function were studied. Harmine did not alter the basal levels of secretion at 2.8 mM glucose. Similarly, stimulated secretion levels were maintained in harmine treated islets, reflecting unaltered β-cell function. However, harmine induced a significant reduction in the levels of total content (Figure 1).
Detection and Quantification of Proliferating β-Cells
In order to probe the effect of target compounds on the β-cell population within pancreatic islet microtissues, it is necessary to be able to quantify that sub-population of cells within the tissue and determine whether or not a significant increase in proliferation has occurred. For this, PDX1 (transcription factor associated with β-cell maturation) was identified as the appropriate biomarker label to detect β-cells and anti-Ki67 was used for labeling the proliferating sub-population along with DAPI to counterstain for all nuclei. The population of proliferating β-cells as well as total proliferating cells within each islet microtissue were then quantified by colocalization of the three signals. Both PDX1 and Ki67 are localized within the nucleus of the cell, making identification and colocalization with one another along with the DAPI signal a straightforward analysis.
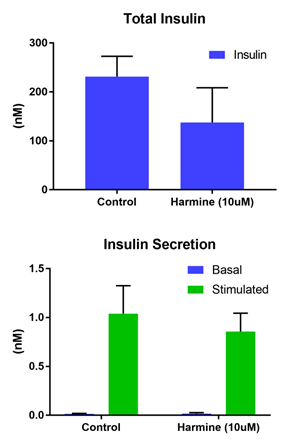
Figure 1. Functional performance in islet spheroids. (Top) Total levels and (Bottom) secreted in basal (2.8 mM) and stimulated (16.7 mM) glucose levels.
The image montages for both control and harmine-treated tissues (Figure 1) show the number of cells positive for Ki67 increases substantially between the two example image sets. A total of 12 tissues for each condition were surveyed such that statistical analysis could be performed and the summarized average total cell counts visualized (Figure 2). The tissues that were treated with harmine on average exhibit a 10-15X fold change increase in proliferating cells (with p-values for Ki67+ only = 10-6, and for Ki67+/PDX1+ = 10-5).
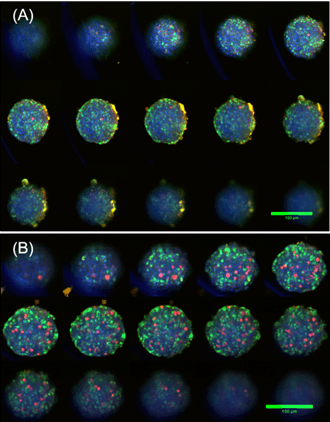
Figure 2. Montages of z-stack images taken from CX7-LZR HCS platform showing individual cells can be counted from each section throughout the tissue, (DAPI = Blue, PDX1 = Green, Ki67 = Red). A) Vehicle control showing typical β-cell distribution in islet microtissue and minimal proliferating cells; B) Harmine (10 µM) treated microtissue showing greatly increased proliferating cell population.
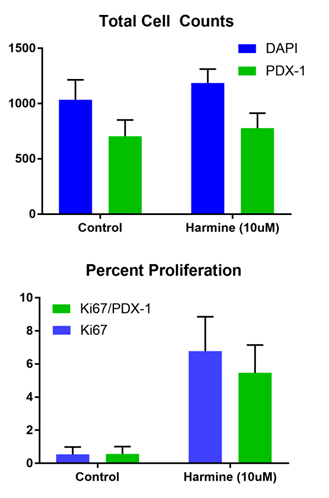
Figure 3. Summary of average cell totals counted for control and harmine-treated microtissues. (Top) Total cell counts are for all cells (blue), and for the β-cell sub-population (green). (Bottom) Percent proliferating cells for all (blue) and β-cell sub-population (green).
The average percentage of proliferating cells for the islet microtissues increases significantly as well, going from ~0.5% proliferating β-cells in the control sample, to ~9.8% proliferating β-cells in the harmine-treated samples, consistent with what has been observed previously4. The distribution of proliferating cells within each microtissue can be elicited from the high-content imaging data, showing that the proliferating population is dispersed relatively uniformly throughout the tissue, with cells on the interior having equal chance of being affected by harmine as those on the periphery.
When conducting confocal imaging of microtissues without clearing, only the outermost cells are detected due to light scattering that reduces signal to noise. Use of a tissue clearing agent greatly increases the number of cells detectable by high content imaging and eliminates the need to do time-consuming and technically challenging IHC of microtissues. The non-adherent surface coating of InSphero assay plates was chemically compatible with Visikol HISTO-M. The clearing process takes only minutes and is done within the well plate.
Conclusions
- Using Visikol HISTO-M coupled with confocal imaging, light scattering and out-of-focus light is reduced, allowing for comprehensive profiling of the interior of each islet microtissue.
- Visikol HISTO-M is compatible with immunolabeling and fluorescent staining of microtissues.
- Clearing microtissues with Visikol HISTO-M enables visualization of the interior of microtissues.
- Harmine induces a 10-15X fold change in the number of proliferating cells within 3D InSight™ Human Islet Microtissues, comparable to in vivo results.
Getting Started
1. Email us at info@visikol.com to discuss your research project.
2. Visit www.insphero.com to learn more about pancreatic islet microtissues.
3. Collaborations begin with pilot projects which can then be scaled up according to your requirements.
4. We work end-to-end to help you take advantage of 3D High Content Imaging assays in your workflow.
References
1. LaBarbera, D. V., Reid, B. G., & Yoo, B. H. (2012). The multicellular tumor spheroid model for high-throughput cancer drug discovery. Expert opinion on drug discovery, 7(9), 819-830.
2. Kabadi, P. K., Vantangoli, M. M., Rodd, A. L., Leary, E., Madnick, S. J., Morgan, J. R., & Boekelheide, K. (2015). Into the depths: Techniques for in vitro three-dimensional microtissue visualization. Biotechniques,
59(5), 279.
3. Bell, G.I.B., Polonsky, K.S. (2001). Diabetes mellitus and genetically programmed defects in beta-cell function. Nature 414, 788-791.
4. Wang, P., Alvarez-Perez, J-C., Felsenfeld, D.P., Liu, H., Sivendran, S., Bender, A., Kumar, A., Sanchez, R., Scott, D.K., Garcia-Ocana, A., Stewart, A.F. (2015). Induction of human pancreatic beta cell replication by inhibitors of dual specificity tyrosine regulated kinase. Nat. Med., 21(4), 383-388.

