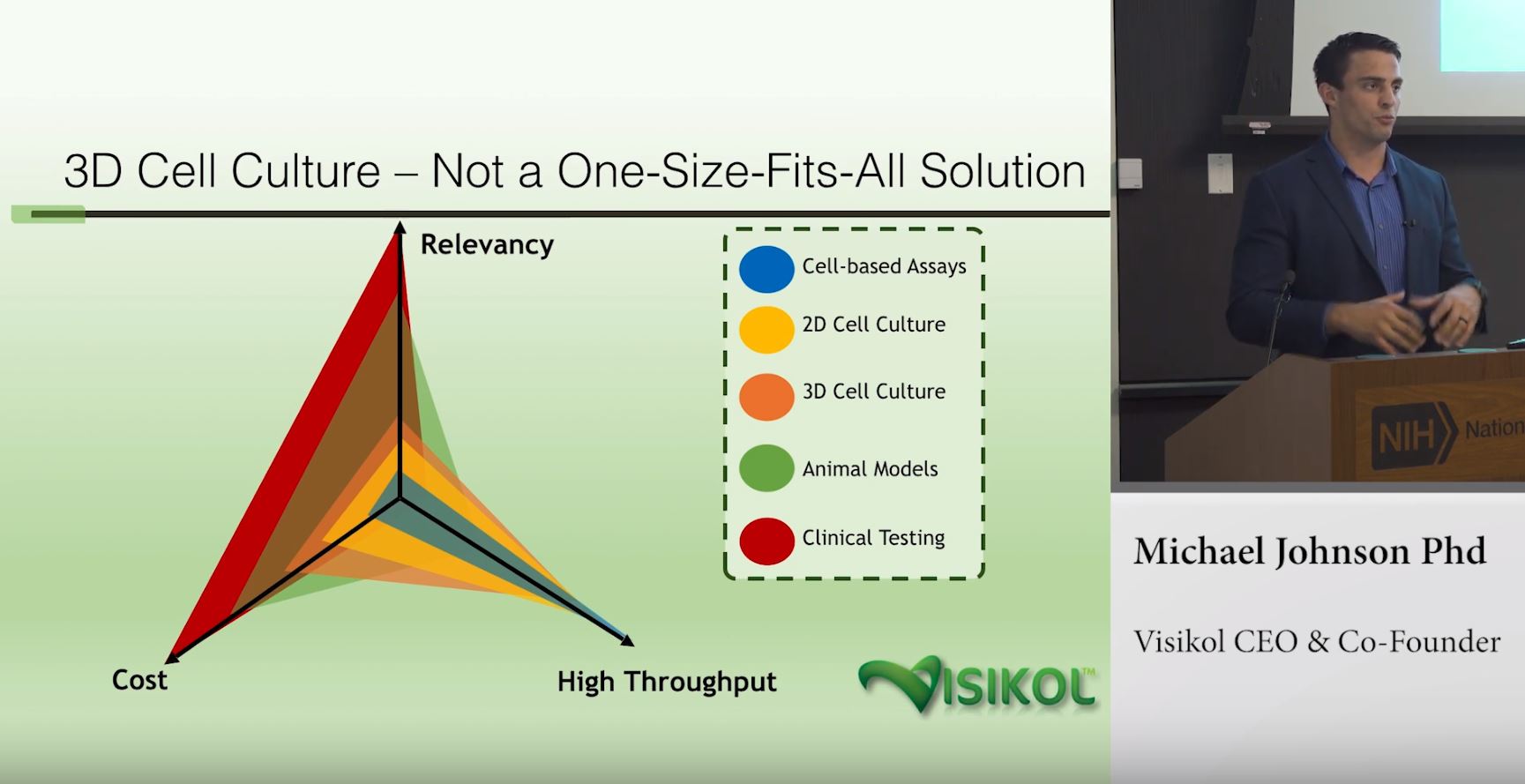
Due to their relatively low cost, higher throughput and continually improving predictive capacity, researchers are increasingly turning to in vitro models for the evaluation of both therapeutic efficacy and toxic liabilities in the liver. While in vivo animal models offer the advantage of evaluating toxicity and disease from a systems-level perspective, in vitro models can often surpass animal model predictive capacity due to the ability to use human cells, which can feature different expression levels of key drug transporters or metabolizing enzymes. In vitro models, and especially 3D cell culture models (CCMs) for the improved relevancy afforded by cell-cell and cell-ECM contacts may offer better success at predicting toxicity and efficacy in the liver. However, choosing the appropriate cells to include in a liver 3D CCM requires careful consideration of several factors including suspected mechanism of action of the therapeutic being evaluated.
The most commonly employed liver 3D cell culture models feature a monoculture of hepatocytes or hepatocyte-derived cells, who’s application can be informative, but limited to studying particular subsets of toxicity mechanisms. Within the monoculture category of 3D CCMs, there exists a variety of options for hepatocyte-like cells, each with unique advantages and disadvantages. For example, HepG2 cells are derived from a hepatocellular carcinoma, and while the nature of this cell line affords ease of implementation for their highly proliferative nature and ease of aggregation in 3D CCMs, these cells exhibit substantially lower metabolizing enzyme expression levels, which ultimately limits their use to the study of parent-compound toxicity, rather than toxicity of drug metabolites, which are most often implicated in drug induced liver injury in vivo.
Terminally differentiated HepaRG cells, which are derived from a human hepatic progenitor cell line, have offered a substantial improvement upon the more traditional HepG2 cells in that their expression profiles of several metabolizing enzymes and drug transporters more closely mimic those of primary hepatocytes than do HepG2 cells. Given their many similarities to primary hepatocytes and their relatively low cost and ease of aggregation in 3D CCMs relative to primary hepatocytes, these cells offer a substantial advantage in screening therapeutics for potential toxic liabilities, especially in stages wherein model consistency is key.
However, the gold standard of toxicity testing, particularly when trying to capture variability in patient populations is primary human hepatocytes. While isolated and cryopreserved hepatocytes have been questioned for their ability to maintain in vivo-like functionality in in vitro assays, improvements in culture techniques as well as quality control designations as to the functional state of each lot qualified have aided in their routine implementation in toxicity evaluation. While pseudo-3D sandwich culture methods have been traditionally employed to improve long-term hepatocyte functionality for in vitro assays, several key technological advancements have aided in the growing popularity of primary hepatocyte implementation in true 3D CCMs (i.e. spheroids, microtissues, etc.). For example, while aggregation of primary human hepatocytes can sometimes present challenges and exhibit lot-to-lot variability, the qualification of specific primary hepatocyte lots as “spheroid-qualified” combined with advancements in microplate coatings (i.e. Corning ultra-low attachment spheroid microplates) have made implementation of primary hepatocyte-centered 3D CCMs more accessible.
Regardless of the hepatocyte or hepatocyte-like cell type chosen, a more predictive in vitro model likely stipulates inclusion of other nonparenchymal cells. For example, as an immune component to liver injury is increasingly being considered as a potential cause of the often-idiosyncratic manifestation of toxic liabilities, inclusion of immunological components such as Kupffer cells can provide more accurate prediction of potential toxic risks. Similarly, presence of other cell subtypes in the nonparenchymal fraction such as stellate cells are critical to modeling certain endpoints of liver injury or disease, such as fibrosis.
Altogether, development of an appropriate liver 3D cell culture model for evaluating toxicity or therapeutic efficacy requires a careful balance of model complexity and cost constraints. Contact Visikol today to learn more about how you can leverage one of several liver 3D CCMs through our OpenLiver platform in the evaluation of general toxicity, cholestasis, steatosis, or fibrosis.