The search for immunotherapies that are effective in their treatment of cancers has been ongoing with varying degree of success. An important aspect of this therapeutic discovery process is the development and application of effective in vitro models for testing such that only efficacious and safe therapeutics make it to expensive animal studies. Visikol, as a leader in in vitro 3D cell culture-based assays recognizes the difficulty and complexity of testing new therapeutics for immuno-oncology applications and has been developing a suite of models and assays specifically for this purpose. In vitro 3D cell culture models are an ideal approach to these assays since they are more biologically relevant than traditional 2D cell culture models and allow for a more accurate assessment of experimental therapies.
Typically, new immunotherapies that are being developed are cellular or antibody-based and to assess these treatment approaches, important questions need to be answered such as
- Are these therapies reaching their target cancer/tumor cells?
- Are the target cells being eliminated either directly or indirectly?
- Are their off-target effects to other types of cells?
Visikol offers a variety of assays to help Clients answer these questions such as Immune Cell Infiltration, Cell Mediated Cytotoxicity/ Effector Function, Antibody Penetration, and Immune Synapse assays. To better understand the services that Visikol offers for immuno-oncology, the following takes a closer look at one of these assays.
Immune Cell Infiltration Assay
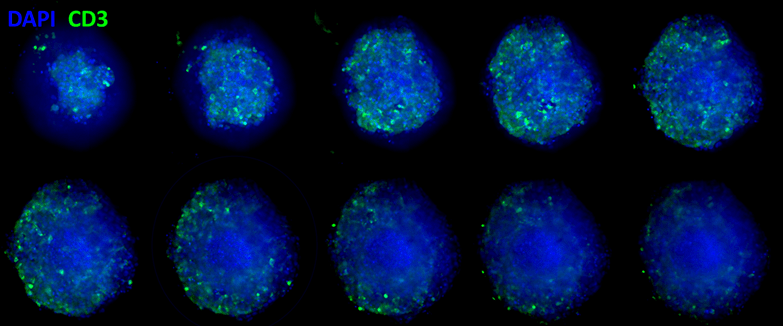
The immune cell infiltration assay uses a 3D cell model (e.g. tumor spheroids) to assess the effects of compounds and immunotherapies on cellular infiltration or to screen immune cell populations for immunotherapies. Various cell types are available to create this 3D model and custom models are available upon request. The test article concentrations and vehicle control are run in triplicates. Concentrations for the test article are run as an 8 point assay (20 ug/mL, 10ug/mL, 2 ug/mL, 1 ug/mL, 400 ng/mL, 200 ng/mL, 100 ng/mL, and 20 ng/mL), and custom concentrations are also an option. Immune cells are labeled with CD3, CD4/CD8 or other immune cell markers through immunolabeling and a DAPI nuclear stain to indicate any cells for an accurate total cell count. Tissues are cleared using Visikol HISTO-M, a clearing reagent that provides high contrast and resolution images of thicker tissues. High content imaging is performed, and images are analyzed. The end points of this assay include: Dose response curves demonstrating the number of infiltrated immune cells vs the test article concentrations; the total number of cells for each group; a distribution of infiltration depth of immune cells; a statistical significance analysis of results in regard to vehicle and treatment groups.
Visikol is committed to assisting its Clients’ in developing novel immunotherapies for cancer treatment and is continually developing novel assays to improve this drug discovery process. Contact us to work with our Visikol team to identify the best assay and model for your specific research needs.