Visikol is a leading provider of advanced cell culture assays and has recently been expanding its portfolio of in vitro assays through the introduction of precision cut tissue slice models. By culturing fresh tissue in an ex vivo format, an unprecedented level of relevancy can be achieved in a medium throughput in vitro assay. As a service provider that is agnostic to the types of models used in its assays, Visikol is continually looking to expand its model portfolio such that every researcher can choose a model which best meets their throughput, relevancy, and cost needs, as no single model is best for all research questions. Through this partnership, Visikol will leverage Melior Discovery’s CCl4-induced mouse liver fibrosis model to provide its Clients with an alternative or supplement to using human-based models in their NASH drug discovery efforts.
Prior to partnering with Melior, Visikol had a robust portfolio of ex vivo human precision cut liver slice models (both healthy F0-F1 and diseased F3-F4) as well as in vitro 3D cell culture models which could all be used to evaluate the efficacy of its Client’s compounds in ameliorating NASH. In one iteration of such models, Visikol can stimulate disease progression in healthy 3D cell culture models or PCLS using TGF-β and/or PDGF-ββ or through a free fatty acid stimulation protocol. While these approaches tend to be slightly higher throughput due to normal tissue availability, to better evaluate efficacy in a disease model that presents the native disease architecture, Visikol can leverage F3 and F4 human PCLS to evaluate NASH amelioration.
The aim of partnering with Melior has been to further expand this suite of NASH models and to provide Clients with an even more diverse model offering. Through a partnership with Melior, Visikol will now be able to provide its Clients with a mouse CCl4 PCLS model of liver fibrosis, wherein liver fibrosis is chemically stimulated in vivo via a carbon tetrachloride injury model. This treatment scheme tends to cause hepatocyte damage, necrosis, inflammation and fibrosis after 14 days of challenge in a manner similar to that seen in normal human fibrotic disease progression.
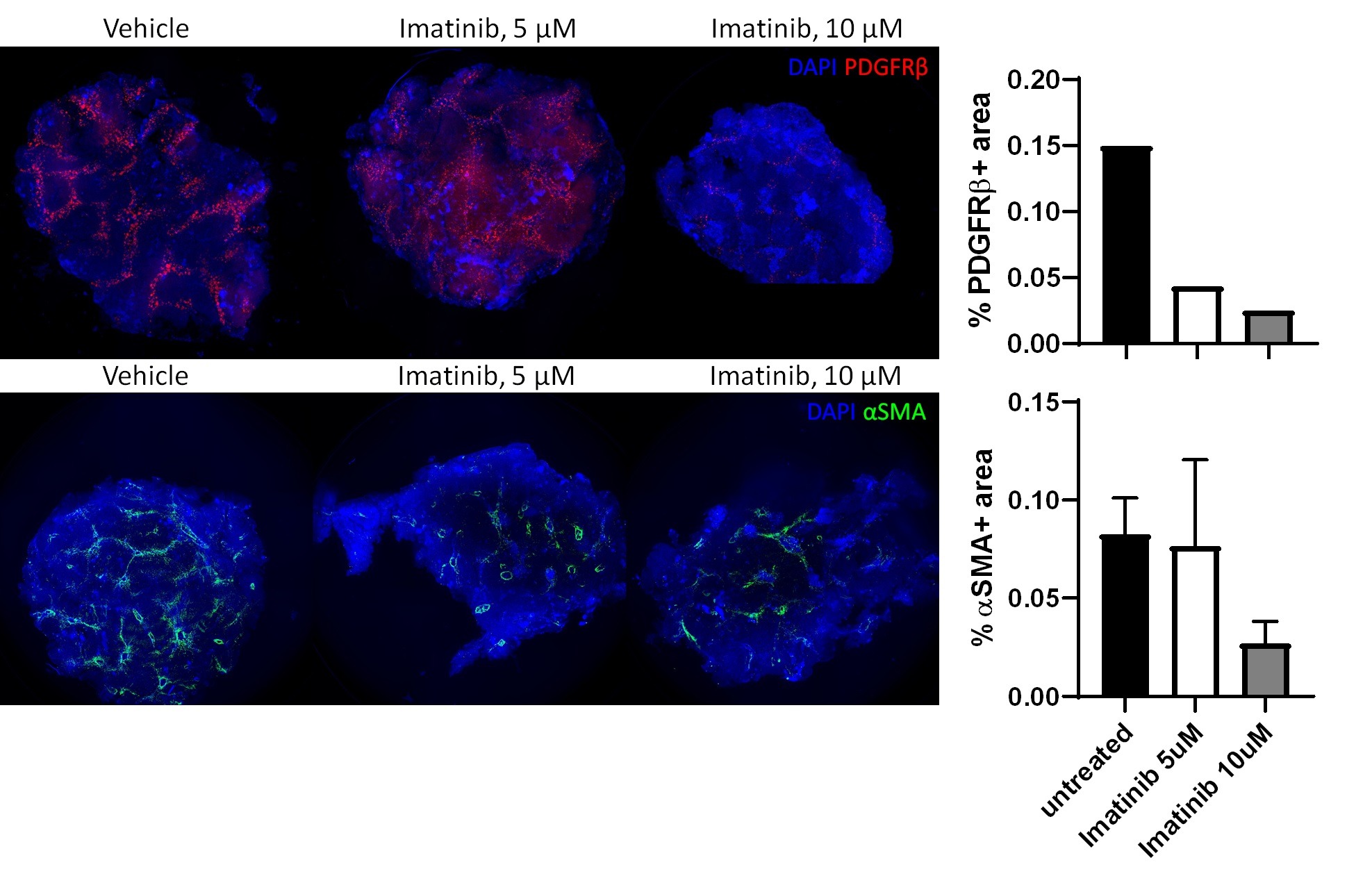
“Since our Clients have targets that are relevant at various stages of NASH disease progression and are looking to evaluate compounds at different stages in the drug discovery pipeline, we find that it is best to provide them with a range of means for assessing their compounds,” described Visikol Head of Services Dr. Erin Edwards. “We’re excited to be able to offer this murine model, as it will go a long way towards helping our Clients achieve their study goals while using a highly reproducible model that is slightly higher throughput than human models, and aligns with 3R principles.”
In comparison to human based PCLS models, this CCl4 mouse model-based approach is highly reproducible, since subjects are from a genetically identical mouse strain and the disease state is induced in a repeatable, laboratory-staged protocol. Additionally, this approach allows for the use of genetically modified models and reduces the overall number of animals required in later stage studies as a single animal is being used to generate multiple PCLS models for analysis. Lastly, this offering enables Clients to conduct comparisons of efficacy between species, facilitating bridging of other preclinical studies.
If you are interested in discussing your NASH program in more detail and learning about the advantages and disadvantages of our different models, please reach out to Visikol today.

