In 2021, over 1,000,000 new cases of cancer and over 600,000 cancer deaths are projected to occur in the United States alone. While analysis of processed cell cultures under a microscope can lead to a diagnosis, a pathologist may request a sample of tissue to further determine cancer diagnosis and progression, known as a biopsy. The understanding of a patient’s biopsy not only aids in the diagnosis and possible treatment routes but can also open the gateway to further understand the progression and the effects of treatment in clinical trial studies. With growing numbers of biopsies per year, more efficient methods of labeling and staining tissues are needed to optimize patient diagnosis, treatment, and clinical trial research. Histological staining and immunofluorescence (IF) for specific biomarkers plays a key role in understanding the patient’s sample fully, however, varied tissue size and incapability to optimize biomarkers and histological staining leads to a loss in vital lifesaving information. This increases the demand for multiplex services which provide labeling with any tumor type, multiple relevant biomarkers, and detailed spatial profiling for a more in-depth understanding of biopsy samples.
The multiplex fluorescence approach can analyze the cells of a single tissue sample with multiple panels of up to four biomarkers per panel expressed on a single cell level with high precision and accuracy. The basic principle of multiplexing is that multiple protein targets are stained by specific antibodies labeled with distinct fluorophores which are imaged using a fluorescent microscope, stripped of all antibodies, and re-labeled for further analysis. With the limited tissue sample size acquired via biopsy collection such as the punch, surgical, and core needle methods, the multiplex approach vastly opens the door for understanding more about the sample compared to other methods while saving tissue samples for further analysis.
For example, a biopsy for breast and prostate cancer commonly collected using a core needle method produces a tissue sample the size of a grain of rice. Once collected, the tissue is assessed for diagnosis, stage/classify if cancerous, and estimate prognosis of cancer. These characteristics are determined by tissue tumor markers which are proteins or other substances that are produced by cancer cells compared to normal cells. These markers can also be utilized to indicate whether a patient is a candidate for a particular targeted therapy and assess treatment efficiency. For example, markers such as estrogen receptor and progesterone receptor are tested to determine whether patients with breast cancer should be treated with hormone therapy, or PDL-1, to see if patients with various cancer types should be treated with an immune checkpoint inhibitor. These mentioned markers and more have been characterized and are used in clinical settings, some of which are associated with only one type of cancer, while others can be associated with multiple different cancer types. With the minuscule size of tissue collected from common biopsy procedures, a multiplex technique opens opportunity to obtain the most information from patient biopsies from prognosis, grade, and even possible routes of treatment.
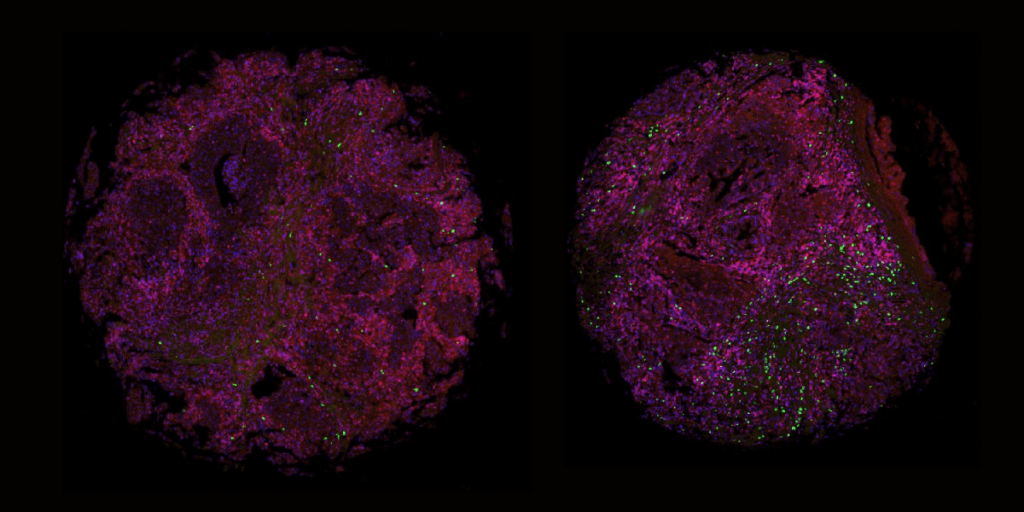
At Visikol, we’ve launched our own fluorescent multiplex approach which allows our team to examine many biomarkers with clinical value simultaneously on a single formalin-fixed paraffin-embedded (FFPE) tissue section, followed by histological staining to assess changes in tissue architecture on the same tissue sample. This provides extensive information regarding colocalization, correlation and spatial relation between various biomarkers, as well as changes to tissue structure on a single biopsy sample. More importantly, these data points enable high dimensional image processing to generate spatial profiles of a patient-level disease microenvironment. This can ultimately provide more accurate prediction, prognosis, and drug treatment.
If you are interested in multiplexing biomarkers and how these operations can excel your research, please reach out to our team to discuss your project. We are always interested to work together with our clients as a team to develop customized assays to best suit their needs.
References
Siegel, RL, Miller, KD, Fuchs, H, Jemal, A. Cancer Statistics, 2021. CA Cancer J Clin. 2021: 71: 7- 33. https://doi.org/10.3322/caac.21654
Taube JM, Akturk G, Angelo M, Engle EL, Gnjatic S, Greenbaum S, Greenwald NF, Hedvat CV, Hollmann TJ, Juco J, Parra ER, Rebelatto MC, Rimm DL, Rodriguez-Canales J, Schalper KA, Stack EC, Ferreira CS, Korski K, Lako A, Rodig SJ, Schenck E, Steele KE, Surace MJ, Tetzlaff MT, von Loga K, Wistuba II, Bifulco CB; Society for Immunotherapy of Cancer (SITC) Pathology Task Force.. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. J Immunother Cancer. 2020 May;8(1):e000155. doi: 10.1136/jitc-2019-000155. Erratum in: J Immunother Cancer. 2020 Jun;8(1): PMID: 32414858; PMCID: PMC7239569.
