Background
Traumatic brain injury (TBI) is debilitating trauma with myriad clinical presentations that can persist for years after the initial injury. Early stratification of the injury and treatment of secondary injury is critical to optimize recovery. A cascade of inflammatory responses in the brain following the initial injury contributes to the severity and progression of the damage over time. The calcium-binding protein S100b has been extensively examined for its role in TBI as a biomarker of acute astroglial injury in addition to a damage-associated molecular pattern. In this experiment, we examined levels of S100b in key areas of rat brains following TBI and in controls using whole-mount immunolabeling paired with reversible, solvent-based tissue clearing, enabling the spatial profiling of S100b as a part of the inflammatory cascade resultant from TBI. Observation of the biomarker S100b using confocal microscopy and 3D reconstruction in relation to astroglial injury in the hippocampus, a critical region of the brain whose increased aggregate of S100b is implicated in several conditions resulting from TBI, allows for the extraction of a significant amount of data from each sample and better visualization when compared to the limitations of traditional 2D histology. Profiling of S100b in 3D will provide an unparalleled, well-rounded understanding of a critical alarmin’s pathway in brain injury. A greater understanding of the role S100b plays in TBI will contribute to the development of more effective therapeutic strategies to mitigate long-term injury from TBI.
Materials and Methods
Tissue in PBS was dehydrated using a progressive gradient of methanol washes at room temperature for 20 minutes per wash. Samples were then rehydrated in the same manner using a gradient of PBS washes before transferring to penetration buffer for one hour. After incubating in blocking buffer at 37 ∘ C for 4.5 hours, a primary antibody dilution of 1:200 of S100b anti-rabbit IgG was used. Samples were incubated in primary antibody labeling buffer overnight at 4∘C . Five 30 minute washing steps each at 37 ∘ C with gentle shaking was used before samples were placed in secondary antibody 1:200 dilution and again afterwards before proceeding to tissue clearing. To clear, tissue was dehydrated and immersed in Visikol® HISTO-1™, and then in Visikol® HISTO-2™.
Traumatic Brain Injury
Current treatments for TBI predominantly seek to mitigate the severity of symptoms, rather than treat the underlying cause of the disease progression and prevent further damage. Often times, the secondary injury resultant from the body’s inflammatory response to the damage causes far more serious complications than the initial trauma. Treatment options offered to patients include diuretics to remove fluid causing increased intracranial pressure, anticoagulants, and analgesics. Antidepressants and other medications may help to ameliorate changes in mood or behavior in addition to therapies for rehabilitation including cognitive behavioral therapy and anger management. S100b inhibitors currently under development may offer an opportunity to prevent secondary injury following TBI in the future.
S100b
S100b is a small, intercellular, calcium-binding protein predominantly found in perivascular astrocytes’ cytoplasm that is highly expressed in the brain, most notably the hippocampus. It is a dose-dependent protein exhibiting neurotoxic and neurotrophic effects. While the body’s normal regulation of S100b has not yet been fully elucidated, the protein is known to be responsible for myriad functions under normal conditions including the regulation of cell proliferation, energy metabolism, and Ca2+ homeostasis. Upon brain injury, S100b is released, resulting in a cascade of inflammatory responses. Increased levels result in neuronal dysfunction and cell death due to inflammatory responses that stimulate astrocyte and microglia via extracellular mechanisms through interactions with RAGE, TLR4, and CD4+ receptors and by activation of ERK1/2 and P53, signaling cytokine release and creating a feedback loop.
S100b is a reliable biomarker in predicting BBB disruption and permeability due to cytokine release resulting from traumatic brain injury. It is more accurate in predicting the severity of a brain injury than the Glasgow Coma Scale in clinical settings. Increased serum levels of S100b after TBI result in serious complications such as seizures, changes in mood or behavior, and progressive neurodegeneration.
An overexpression of S100b is implicated in numerous neurological disorders. Overexpression of HSA21, the chromosomal location of the gene for S100b, as seen in Down’s Syndrome, results in prolonged cell cycle length of neural progenitors or cell death, and increased oxidative stress as seen in Alzheimer’s. In addition, mitochondrial dysfunction or metabolic dysregulation due to increased levels of S100b results in EEG abnormalities in the hippocampus and seizure activities. Other diseases exhibiting increased serum levels of S100b include Parkinson’s, Epilepsy, PTSD, Multiple Sclerosis, Schizophrenia, and certain cancers.
Imaging
Samples were imaged using a Thermo Scientific Cellnsight CX7 LZR High-Content Screening (HCS) confocal microscope. Z stacks of each tissue sample were collected DAPI laser and emission filter and 488nm laser and FITC filter. FIJI (Image J) was used to edit each image stack.
Contributing Authors: Kathryn Bozell – St. John’s University and Jeffery Goodman CUNY, Staten Island
Perimortem injured brain stained with DAPI
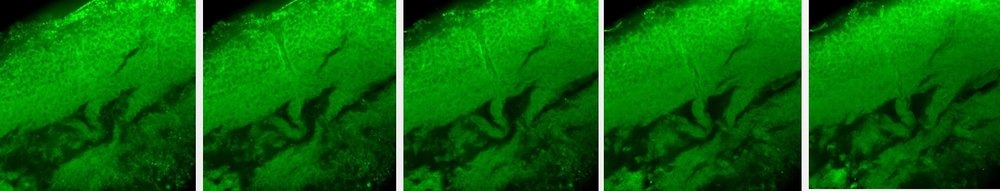
Control brain stained with S100b antibody
