In the last few years, the application of tissue clearing and molecular labeling approaches for the for the fluorescent labeling and 3D imaging of tissues have dramatically increased and with this increase researchers need to acquire this data from their tissues. While researchers have been able to render tissues transparent and stain them, we receive a significant number of questions about tissue imaging and how to obtain 3D imaging data from tissues. Here, we provide background on how best to use confocal microscopy to image tissues in 3D and a brief background on confocal microscopy.
Terminology
- Upright Confocal: Objectives are mounted above the stage – ideal for dipping objectives.
- Allow for a substantial imaging depth into tissues.
- Limited in imaging cell cultures and some well-plate format tissues.
- Inverted Confocal: Objectives mounted below the microscope stage.
- Ideal for well plates.
- Air or oil objectives can be used.
- High Content Confocal: Typically an inverted confocal that is designed for high throughput assays on well plates. These microscopes are essentially inverted confocal microscopes in a box designed for high throughput assays and controlled environment conditions.
Examples of high content confocal microscopes include:
Overview
- Best utilized for high resolution imaging of 3D tissues. Lower resolution and higher volume imaging is best with light sheet microscopy.
- High content confocal microscopy can be automated for high-throughput analysis.
- Ideal for < 2mm thickness tissues.
- Performance highly dependent upon objectives.
- Due to rastering each pixel by laser scan, 1024×1024 image requires ~1-2 second scan time
- Long scan times required for thick tissue.
- Each x-y coordinate scanned once for each Z step – can induce photobleaching in thick samples.
Diagram: Confocal Microscope Light Path
The concept of how confocal microscopy actually works is relatively simple. The specific functionality and features of each system will vary but here’s a general overview: An excitation laser or LED light source is focused to a narrow beam of light that is passed through the microscopes’ objective and through a clear tissue such as a mouse brain described in the diagram. While there are many confocal microscopes that use LED light to reduce instrument cost, laser excitation is preferred for deep imaging into tissues. As the excitation light source passes through the cleared and labeled tissue, it will cause all the fluorophores in its path to fluoresce at a different wavelength than the excitation wavelength. This fluorescence is then captured by the microscopes objective and pass through a dichroic mirror which reflects the excitation wavelength that allows the emission wavelength to pass through.
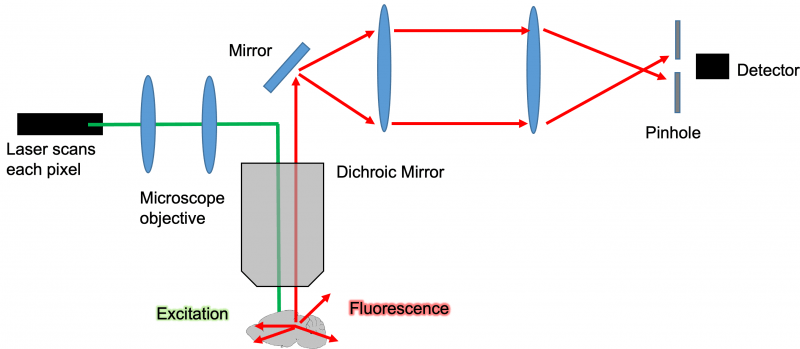
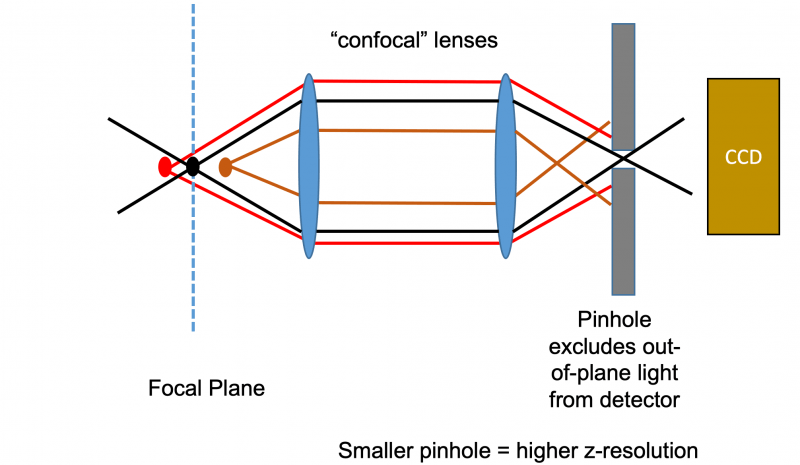
Principle of Confocal Pinhole Element
The magic of the confocal microscope all comes down to its pinhole mechanism that differentiates it from a standard EPI fluorescent microscope. Without the pinhole, all the fluorescent light from the entire depth of the tissue would reach the detector and the image one would see would be a very blurry composite of all the planes within the tissue stacked on top of one another. The pinhole uses confocal lenses to focus the light that is captured from a tissue through a very narrow pinhole. This pinhole mechanism works by allowing to pass through that is only in the focal plane of the microscopes’ objective. The pinhole will therefore reject out plan light and allows a microscope of optically section tissues and acquire stacks of optical Z slices.
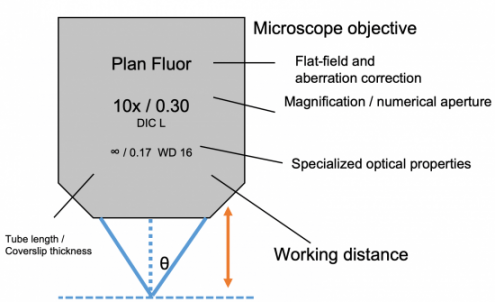
Basic Principles of Microscopy
- Working Distance: Distance from objective to focal plane. You cannot image a tissue thicker than 2X your WD (since each side can be imaged up to WD).
- Field of View: Amount of object that can be viewed using eyepiece and objective combination. Depends on field number of eyepiece/total magnification.
- Numerical aperture (NA): Number characterizing range of angles of light from focal plane that will enter the objective. Higher values = more light entering lens.
Maximum NA for air objectives is 1. Higher NA achievable with immersion objectives
Refractive Index: Dimensionless number that describes how light bends as it passes through a medium. Mismatches in refractive index between an objective, imaging medium, and tissue will result in significant imaging artifacts and this effect is worsened by imaging depth. For optimal depth imaging it is suggested that the RI of an objective is matched with the imaging medium and tissue RI.
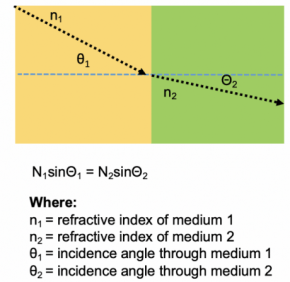
When imaging tissues, it is very important to minimize refracted index mismatches as it will cause imaging artifacts which increase with depth. Microscope objectives are designed to perform at specific refractive indices and are matched to common mediums such as air, water, glycerol, or oil. Some objectives will have correction collars so they can be used with many different refractive indices. For example, ideally an objective that is matched to a refractive index of 1.33 for water will be dipped in water and used to image a tissue that has been cleared with an aqueous clearing technique where the refractive index is also 1.33. When imaging the tissue, try to always match refractive indices for optimal imaging depth and image quality.
For additional questions, email us at info@visikol.com
