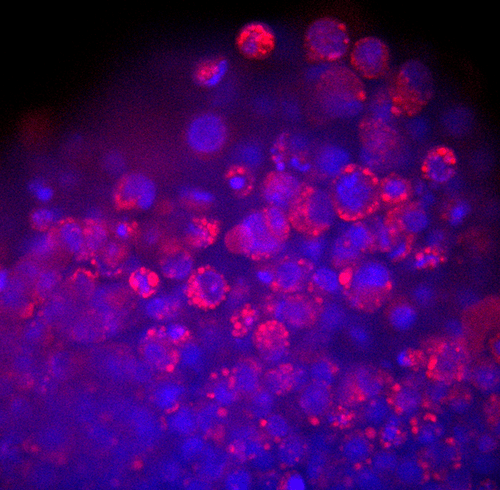
Toxicity and Toxicology
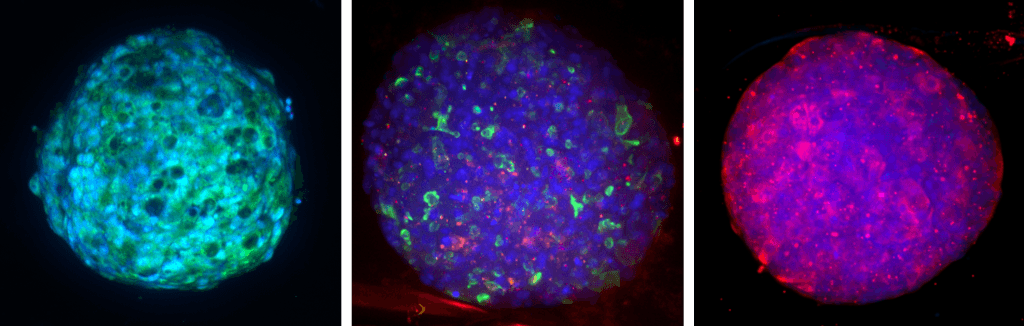
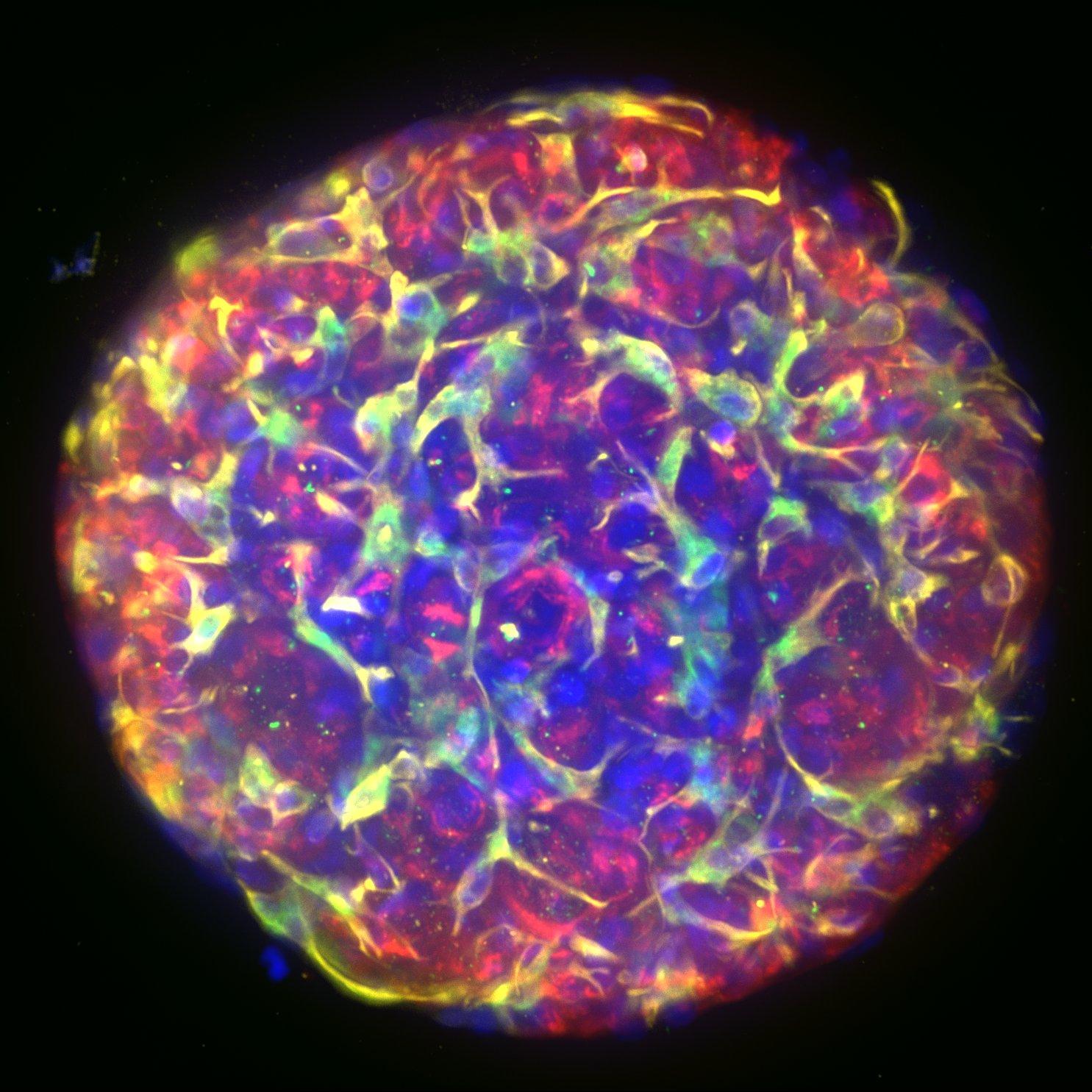
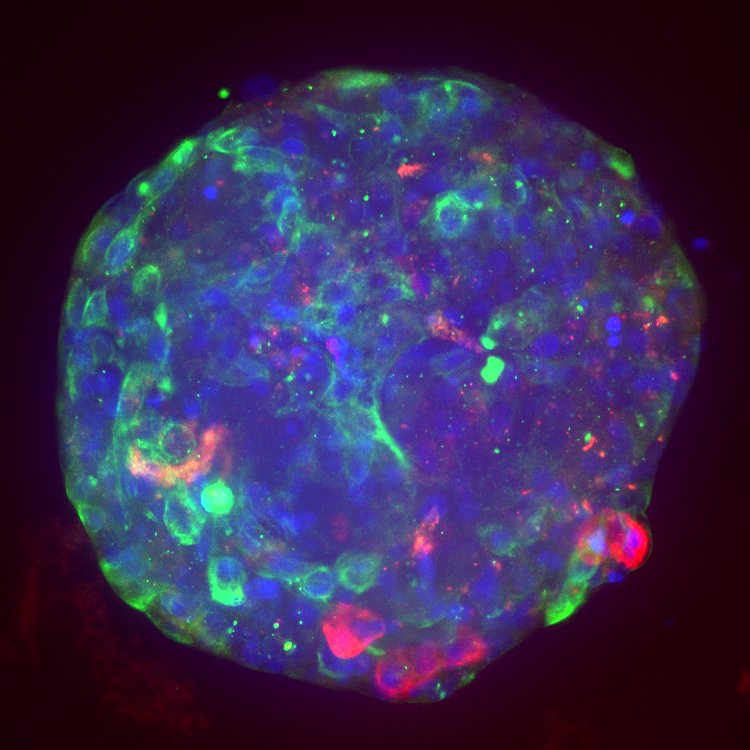
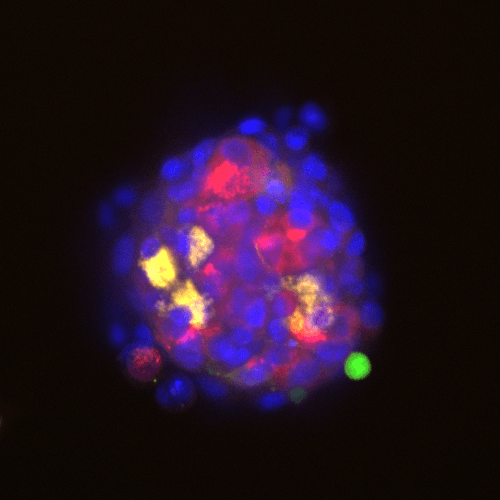
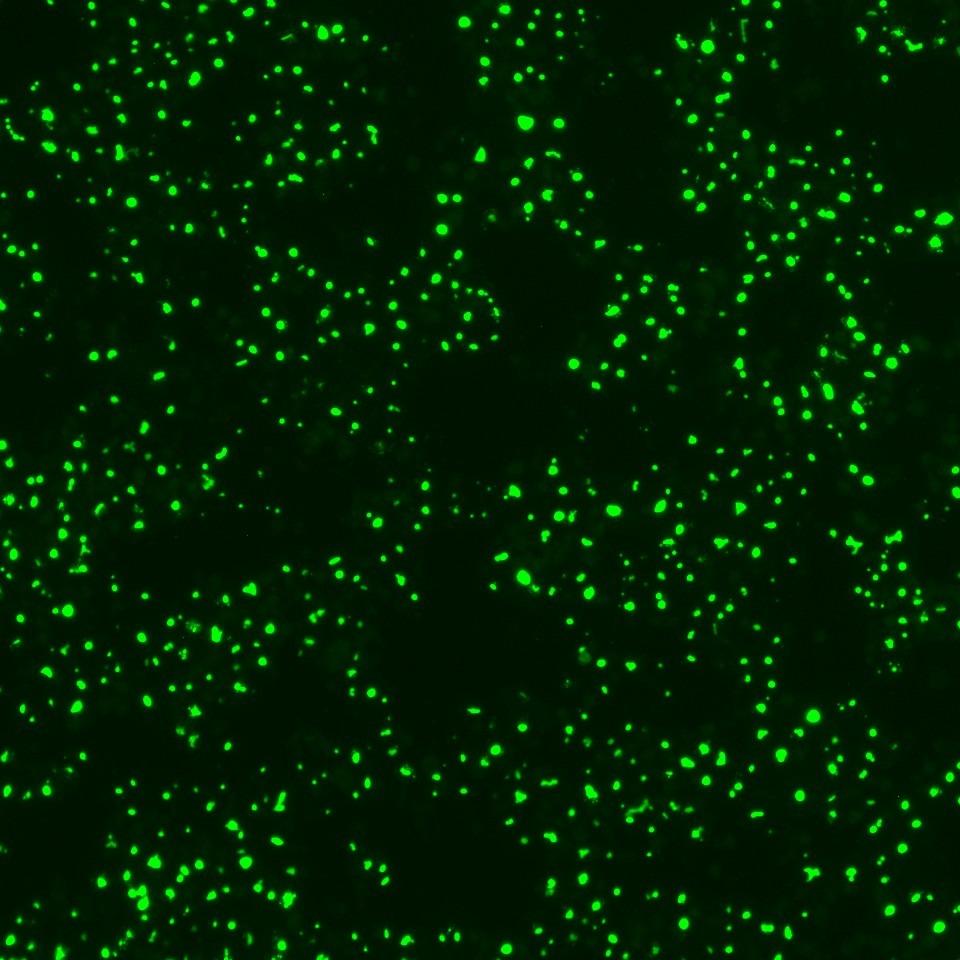
Adverse drug reactions account for the majority of late clinical phase and post market withdraws, which translates not only to lost revenue, but, more importantly, increased morbidity and mortality in patient populations. Drug induced liver injury (DILI) in particular represents the most probable cause for toxicity-related withdrawal of a drug from the market, likely due to first-pass metabolism effects. While in vivo toxicity studies often lack clinical translatability due to differences in expression of key drug transporters and metabolising enzymes between species, humanized in vitro cell culture models have offered promise in filling in these gaps.
At Visikol, we have developed and validated a number of primary cell- and cell line-based 3D cell culture models and assays to evaluate toxic liabilities of our Clients’ compounds in the liver as well as in other common sites of toxicity (i.e. neurotoxicity, cardiotoxicity, etc). Moreover, using functional endpoints multiplexed with advanced imaging and image analysis approaches, we’re able to tease out dose-dependent effects and develop a mechanistic understanding of various modes of cytotoxicity. We work closely with our Clients to ensure the most appropriate and informative model and endpoints are leveraged to provide our Clients with confidence in the translatability of these results to the clinic.