Background
- The blood brain barrier (BBB) behaves as a highly selective semipermeable membrane separating circulating blood from the central nervous system while allowing the passage of glucose, water, and amino acids into the cerebrospinal fluid [1].
- The BBB protects brain nervous tissue from the fluctuation of plasma composition, from pathogenic agents, and maintains homeostasis of the brain parenchyma by restricting non-specific flux of ions, peptides, proteins and cells into and out the brain.
- The BBB is permeable to hydrophobic molecules and utilizes active transport to move ions, glucose, and other critical molecules across the membrane. When functioning properly, the blood brain barrier is a complex hurdle that must be overcome when delivering drugs to the brain.
- Assessment of BBB permeability of drug compounds is critical for drugs targeting regions of the brain; many drugs developed to treat Central Nervous System (CNS) disorders are unable to reach the brain parenchyma in therapeutically relevant concentrations.
- Poor penetration of the BBB is the cause for attrition for 95% of drugs developed for neurological disorders. As such, it is of great interest to explore potential molecules that can modulate BBB permeability [2].
- Disruption of the BBB is observed in many neurological disorders, including multiple sclerosis, stroke, Alzheimer’s disease, epilepsy, and traumatic brain injury, and is frequently induced by neuroinflammation [1].
- The BBB is a complex system, which is difficult to mimic in vitro, which typically had to be addressed with costly, low-throughput animal studies.
- Utilizing a novel BBB in vitro model, we offer an in vitro assay capable of assessing the penetration kinetics of molecules passing across the BBB.
- We have partnered with FUJIFILM to use their iCell Kit.
- Additionally, we offer a complementary assay to assess modulation of BBB permeability due to drug treatment.
- This assay service allows for both compound transport across the barrier to be studied as well as the effect of compounds on the structure and function of the BBB.
General Procedure
- 3D Blood Brain Barrier models are cultured over 4 days to establish a viable barrier as measured by transendothelial electrical resistance (> 150 Ω x cm2). On the fifth day, the models are dosed with test articles for twenty-four hours.
- If assessment of alterations to BBB permeability due to drug treatment is required, a four-compound control panel is added to wells.
- Media is sampled for quantitation of test articles by LCMS/MS.
- Apparent permeability is calculated.
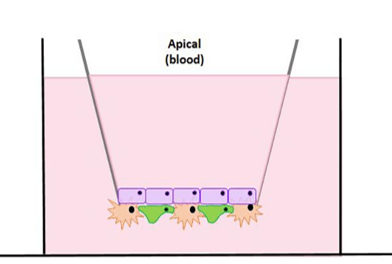
Figure 1. Brain Microvascular Endothelial Cells (Blue) are cultured on the top of the transwell and Pericytes and Astrocytes are cultured (Orange and Green) on the bottom of the transwell to form a barrier that mimics an in vivo model.
Protocol
| Instrument | (LC-MS/MS) system composed of an Agilent 1100 LC coupled with a SCIEX QTRAP 4000 |
| Analysis Method | LCMS/MS Plate Reader |
| Markers | Four-Compound Control Panel
|
| Cell model | Human Blood Brain Barrier Model.
|
| Time points | 24 hours after dosing (custom time points available) |
| Test Article Concentration | Single point assay (1 µM) (custom concentrations available) |
| Number of Replicates | 3 replicates per time condition |
| Quality Controls | Four-Compound Control Panel |
| Test Article Requirements | 50 uL of 20 mM solution or equivalent amount of solid |
| Data Delivery | Apparent Permeability of test compounds in either the apical or basal direction Change in Apparent Permeability of BBB due to effect of drug compound (optional) |
Validation Data
In Vitro vs In Vivo Outcomes
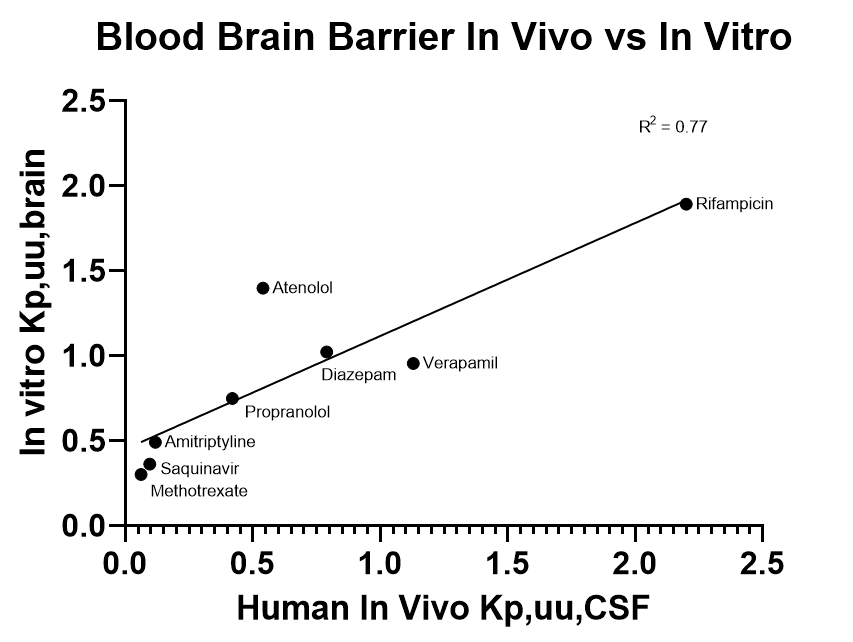
An in vitro model is only as useful as its ability to predict in vivo outcomes. With a handful of control compounds, we are able to demonstrate a positive relationship between the apparent permeability seen within in vivo systems to in vitro models.
Control Compounds
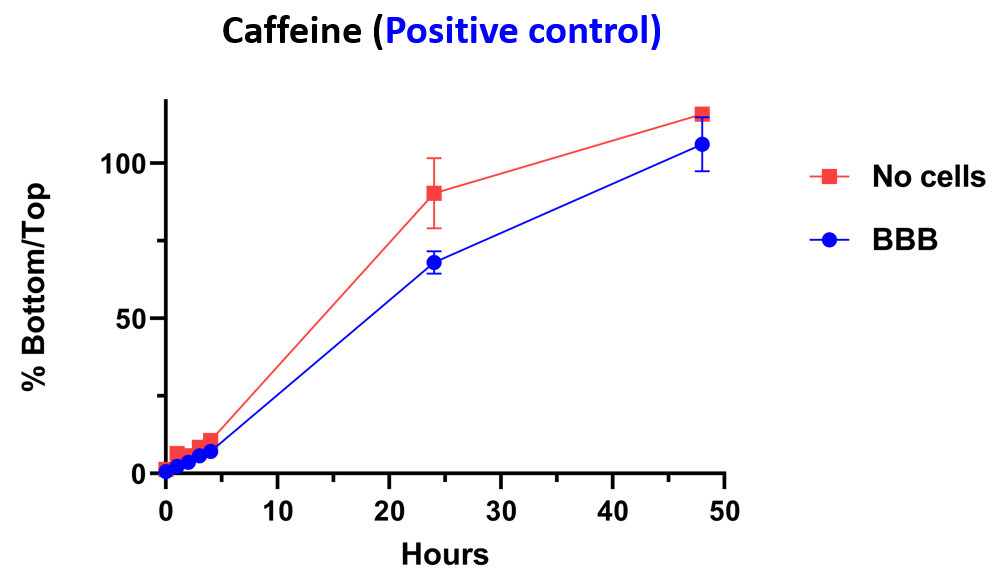
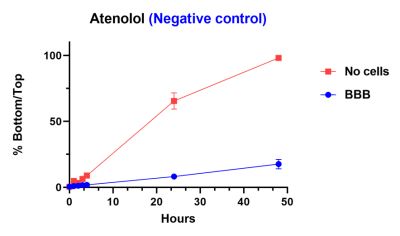
Within this model, we have demonstrated that caffeine (positive control) is able to pass through barrier whether or not the cells are present. A negative control is blocked from passing through barrier by the inclusion of the cells.
Reference Compounds
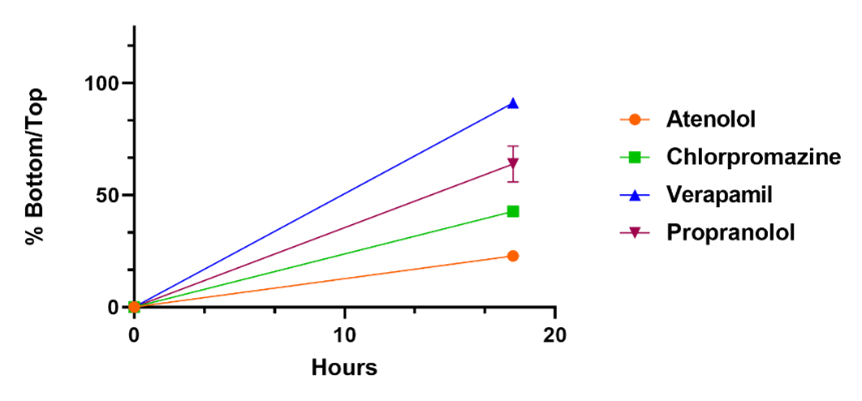
Reference compounds are used to compare differences in compound penetration across the BBB as a function of time. 100 % indicates the compound has reached equilibrium across the membrane.
Barrier Integrity
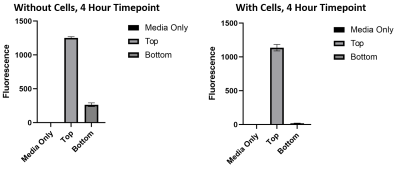
3kDa fluorescently conjugated dextran is able to pass the barrier when cells are not present, however when cells are present dextran molecules are not able to pass through.
Compound Performance
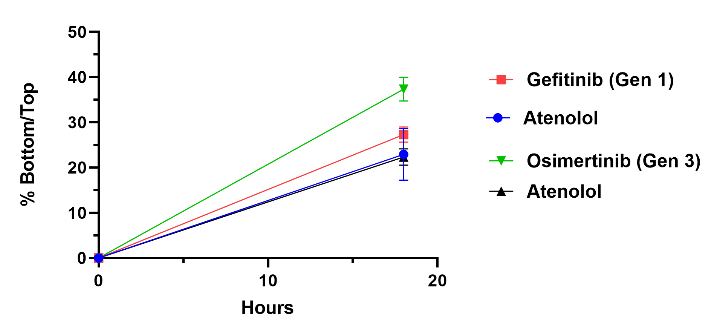
Two EGFR inhibitors a Generation 1, Gefitinib, and a Generation 3, Osimertinib were tested in our model and analyzed through LCMS/MS. Both are used to treat non-small cell lung carcinoma along with other cancers. Osimertinib shows better penetration across the BBB when compared to Gefitinib as well as our negative control, Atenolol.
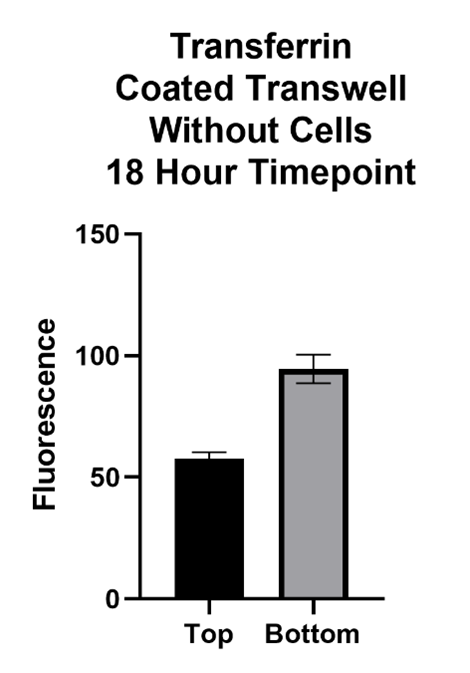
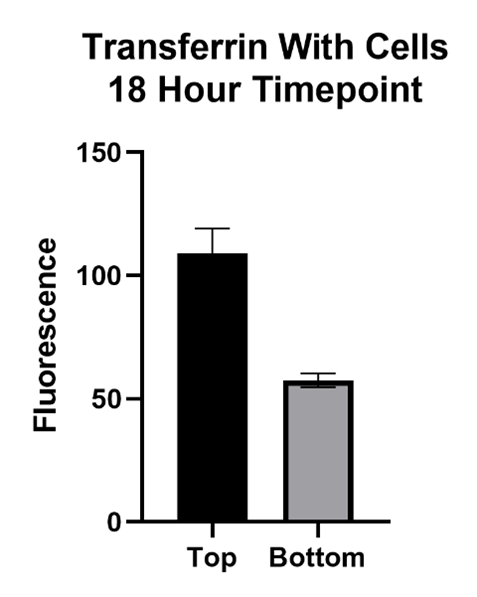
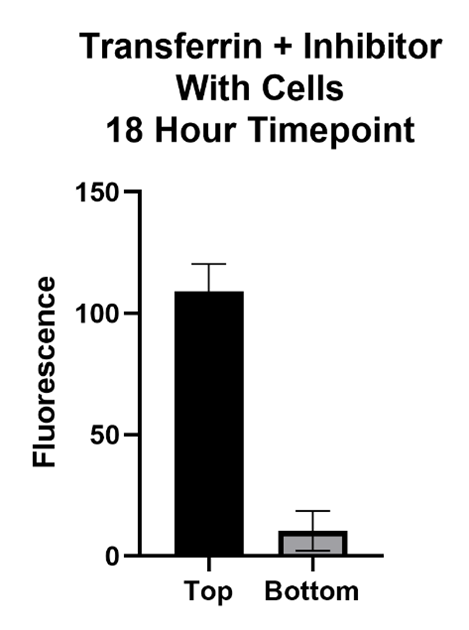
Large Molecule Performance
The Transferrin protein is of interest because of its form of transport across the BBB. This molecule passes the BBB via receptor mediated transcytosis. The transferrin protein binds to the transferrin receptors on the barrier which carry it across. The inhibitor, Ferrostatin II, is a compound that binds to the Transferrin receptor, blocking the Transferrin protein from binding and being carried across.
References
- Daneman, R. (2012). The blood–brain barrier in health and disease. Annals of neurology, 72(5), 648-672.
- Bourassa, P., Alata, W., Tremblay, C., Paris-Robidas, S., & Calon, F. (2019). Transferrin Receptor-Mediated Uptake at the Blood–Brain Barrier Is Not Impaired by Alzheimer’s Disease Neuropathology. Molecular pharmaceutics, 16(2), 583-594.
