Multi-Parameter Toxicity (HUREL Tox™)
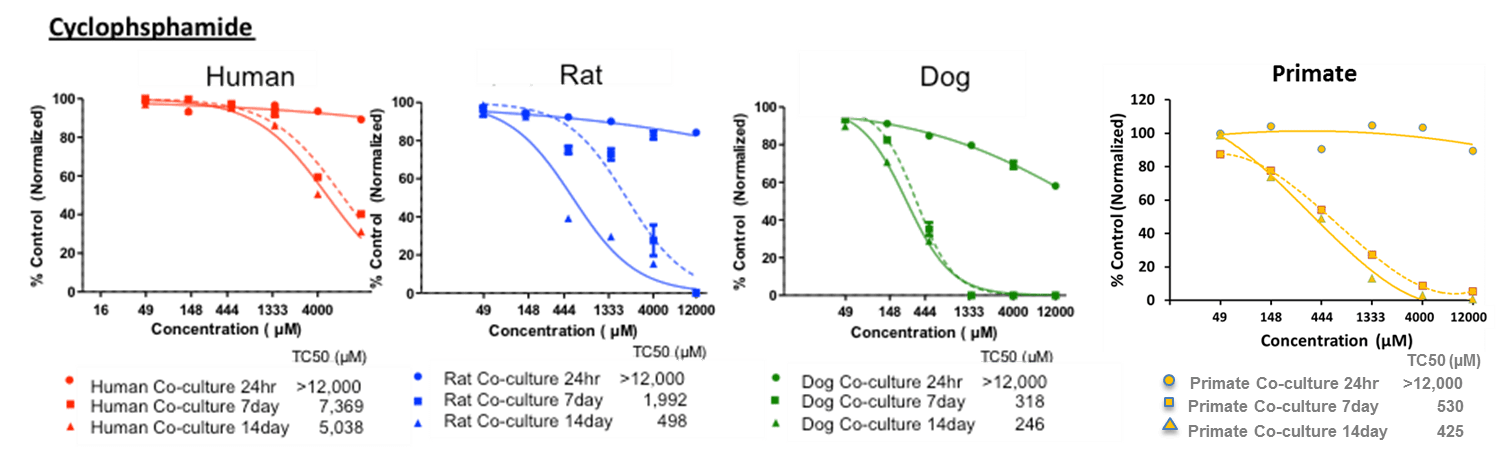
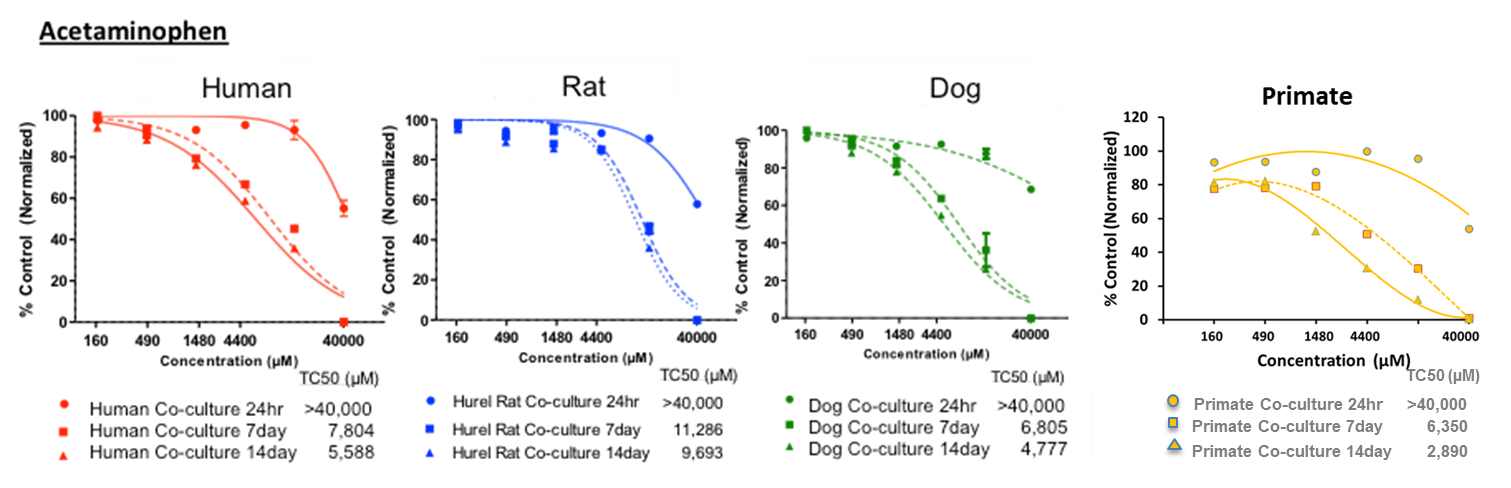
Our Multi-Parameter Toxicity assay’s apply HUREL’s superior metabolic competency to a panel of complementary assays, creating a multi-parametric characterization of hepatotoxic risk. HUREL Tox™ incorporates a 14-day, repeat-dose cytotoxicity study with both ATP and albumin (ELISA) readouts. Computation of time-based HUREL ratios™ and ACEA RTCA impedance signal indices illuminate potential chronic liabilities of pre-candidate NME’s. Incubation in the absence and presence of broad CYP inhibitor aminobenzotriazole (ABT) reads on the potential for formation of reactive metabolites. Incubation in the absence and presence of bile salts provides a marker of potential cholestatic liability.
When provided as a contract research service, the HUREL Tox™ assay panel can be custom-configured according to the client’s particular study design.
Protocol
14-day, repeat-dose cytotoxicity study. Readouts include:
· ATP – 24 hr, 7-day, 14-day
· Agilent xCELLigence® real-time cell analysis monitoring
· +/- Bile Salts
· +/- ABT
· ALT
Models Available
Study Turnaround Time
Receipt of sample to data delivery,
is 4-5 weeks
Example Results
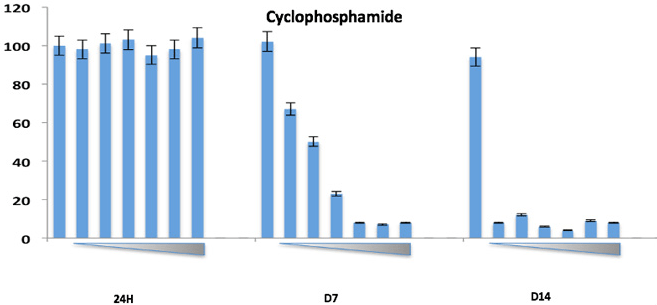
ATP-24hr, 7-day, 14-day
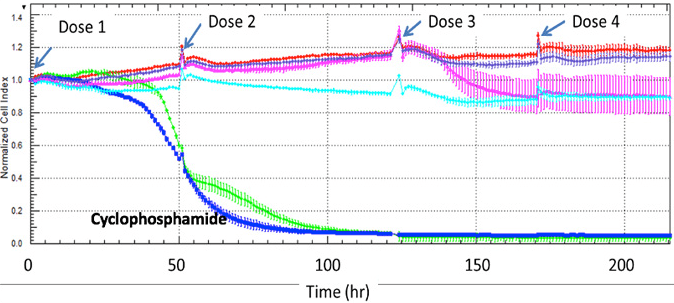
Agilent xCELLigence® real-time cell analysis monitoring
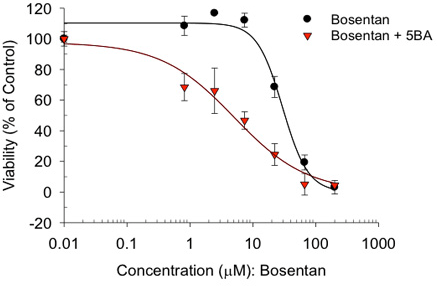
+/- bile salts
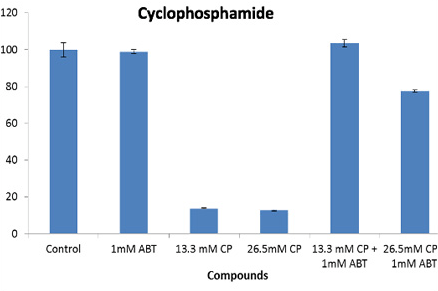
+/- ABT
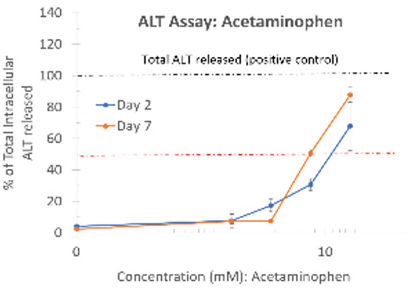
ALT