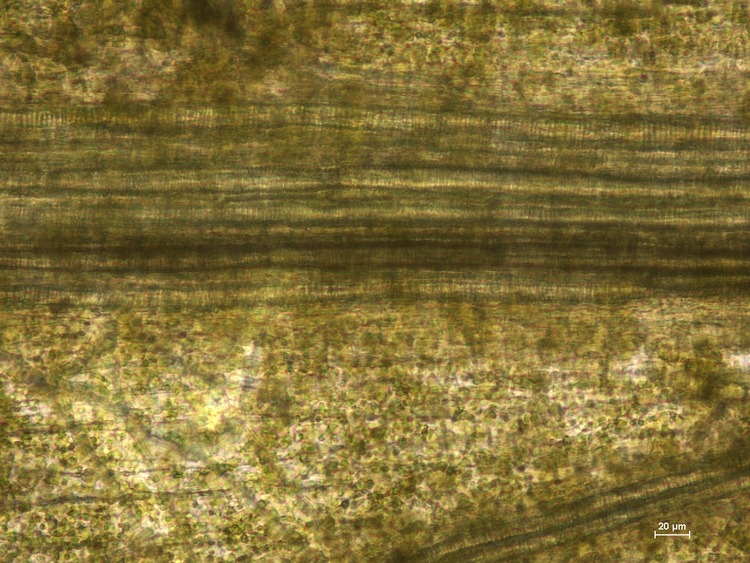
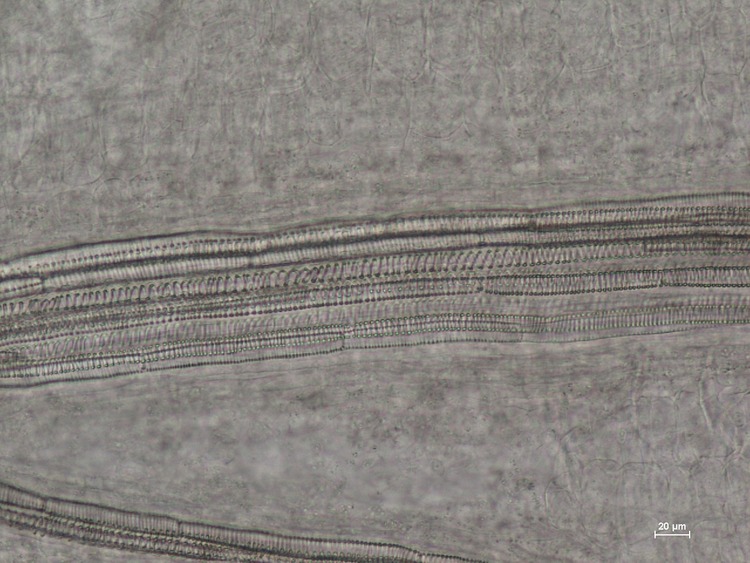
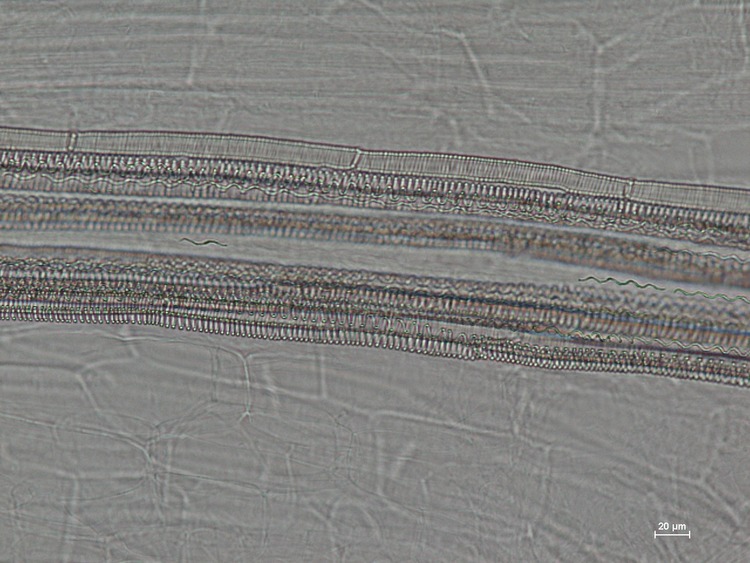
Visikol® for Plant Biology™ is a reagent designed to replace traditional plant tissue clearing in a wide variety of microscopic applications. Here, the superior clearing action of Visikol for Plant Biology on Arabidopsis thaliana can be easily observed when directly compared to traditional plant tissue clearing.
See also: Visikol for Plant Biology Peer Reviewed Paper
Introduction
Microscopic observation has been long used by scientists conducting biological research. In plant science, botanical pathology, and anatomy, some unique challenges are encountered when attempting to observe cells under the microscope. Most tissues contain pigments or other opaque structures, and so they require a clearing procedure to improve visualization. Also when light passes through optically clear tissues and organelles, the different refractive indices of the materials can scatter the light and severely reduce the clarity of the image, obscuring the internal structures of interest. So in order to get the best image possible, the tissue is treated with a clearing agent to both remove the internal pigmentation and improve the refractive indices of the different components of the tissue.
With advances in optical imaging, the utilization of clearing agents has allowed scientists to capture incredibly detailed high-resolution images (Haseloff, 2003).
Pharmacopeias like the US Pharmacopeia, American Herbal Pharmacopoeia, and the WHO have published procedures for microscopic authentication of herbal preparations using Hertwig’s solution. And consequently, Hertwig’s solution has become the industry standard used daily in laboratories focused on the quality assessment of herbal products.
However not everyone can obtain the materials to make Hertwig’s solution. In the U.S., one of the main components of Hertwig’s solution is a Schedule IV substance, controlled by the Drug Enforcement Administration. In addition to high yearly permit fees, the DEA requires detailed documentation of every transfer and use to ensure no material is illegally diverted. The compliance cost of these regulations Hertwig’s solution impractical for the large majority of users in the field, and as such, their ability to perform most accepted standard techniques is limited. This restriction leads to substandard quality control in industry, and constricts both research and educational opportunities.
Visikol for Plant Biology has been reported in the scientific literature as a suitable, non-regulated substitute for Hertwig’s solution in microscopic applications for botanical and agricultural quality assessment, pathology and histology, research as well as for teaching (Villani, 2013).
Materials and Methods
The control solution of Hertwig’s solution was created using standard methods. The refractive index for each chemical was determined using a temperature controlled refractometer at 23°C (Fisher Scientific Model #: 334620). The refraction index of Visikol for Plant Biology (1.4450) was higher than Hertwig’s solution, lactic acid, ethanol and water (Table 1).
Seven day old, dried Arabidopsis thaliana (L.) Heynh. (Brassicaceae) seedlings were submerged in Visikol for Plant Biology, Hertwig’s solution, or water for 30 minutes. Specimens were placed on a microscope slide (Fisher Scientific, Cat No. 12-544-1, 3”x1”x1mm) and mounted either with two drops of Visikol for Plant Biology, Hertwig’s solution (control), or water and a cover slip (Fisher Scientific, Cat. No. 12-548-B, 22×22-1, 0.17 mm thickness) was put over each. Slides were then heated on a hot plate (60-80°C) for 30-60 seconds until just before boiling, when the air bubbles moved out to the edges of the slide. Each sample was replicated three or more times. All the microscopic image analyses were taken on a Nikon eclipse 80i microscope, with NIS D 3.00 SP7 imaging software (Nikon, Tokyo, Japan). Results are shown below in Figure 1.
Table 1. Table of Media by Refractive Index
| Medium | Refractive Index |
|---|---|
| Water | 1.3330 |
| Ethanol | 1.3550 |
| Hertwig’s solution | 1.4280 |
| Visikol for Plant Biology | 1.4540 |
Conclusions
The images presented here show that Visikol for Plant Biology can be effectively used as a direct replacement of Hertwig’s solution in botanical microscopy. Visikol for Plant Biology yields high quality microscopic images and can be used to clear herbal products for research, quality assessment and botanical authentication. Treatment with Visikol for Plant Biology clears tissues and, due to the increased depth of field over Hertwig’s solution, it allows different layers of internal structures as well as surface details of the specimen to be simultaneously identified, without the need for sectioning or remounting. As these results show, Visikol for Plant Biology is the superior, non-regulated alternative to Hertwig’s solution for use in research, education and quality control.
Figure 1. Arabidopsis thaliana images
Table 2. Overview of literature which utilizes Hertwig’s solution to clear specimens
| Title | Specimens | |
|---|---|---|
| McBryde, 1936 | A Method of Demonstrating Rust Hyphase and Haustoria in Unsectioned Leaf Tissue | Garden beans, cornm mayapple and barberry |
| Arnott, 1959 | Leaf Clearings | Syringa sp., Crossosoma parviflorium, Thea sinenss |
| Lersten, 1967 | An Annotated Bibliography of Botanical Clearing Methods | Bryophytes; "all plant parts, including pollen;" Dalea, Lemna minor |
| Shobe and Lersten, 1967 | A Technique for Clearing and Staining Gymnosperm Leaves | Gymnosperms, Metasequoia glyptostroboides |
| Herr, 1971 | A New Clearing-Squash Technique for the Study of Ovule Development in Angiosperms | Angiosperms, Cassia abbreviata, Ludwigia uruguayens |
| Gardner, 1975 | A Overview of Botanical Clearing Techniques | Review paper |
| Lersten, 1986 | Modified Clearing Method to Show Sieve Tubes in Minor Veins of Leaves | Soybeans and other dicotyledonous species |
| Jackson and Snowdon, 1990 | Atlas of Microscopy of Medicinal Plants, Herbs, and Species | >100 common herbs and spices |
| Herr, 1993 | Clearing Techniques for the Study of Vascular Plant Tissues in Whole Structures and Thick Sections | Wisteria sinensis, Selginella apoda, Abelia grandiflora, Nymphaea odorata |
| Liang and Herr, 1994 | Use of the Four-and-a-Half Clearing Technique to Study Gymnosperm Embryology: Cunninghamia lanceolata | Cunninghamia lanceolata |
Work Cited
- Arnott, H.J. 1959. Leaf clearing. Tutox News 37: 192-194.
- Gardner, R.O. 1975. An overview of botanical clearing techniques. Stain Technology. 50:99-105.
- Haseloff, J. 2003. Old botanical techniques for new microscopes. BioTechniques 34: 1174-1182.
- Herr, J. M, Jr. 1971. A new clearing-squash technique for the study of ovule development in angiosperms. American Journal of Botany 58: 785-790.
- Herr, J. M. Jr. 1993. Clearing techniques for the study of vascular plant tissues in whole structures and thick sections. In C.A. Goldman, P.L. Hauta, M.A. O’Donnell, S.E. Andrews, and R. van der Heiden, Editors. Tested studies for laboratory teaching. Volume 5,63-84. Proceedings of the 5th Workshop/Conference of the Association for Biology Laboratory Education (ABLE).
- Jackson, B.P. and D. W. Snowdon. 1990. Atlas of microscopy of medicinal plants, culinary herbs and spices. Belhaven Press, London.
- Lersten, N.R. 1967. An annotated bibliography of botanical clearing methods. Iowa State Journal of Science 41: 481-486.
- Lersten, N.R. 1986. Modified clearing method to show sieve tubes in minor veins of leaves. Stain Technology 61:231-234.
- Liang, D. and J. M. Herr, Jr. 1994. Use of the four-and-a-half clearing technique to study Gymnospermem bryoIogy: Cunninghamia lanceolata. Biotechnic and Histochemistry 69: 279-282.
- McBryde, MC. 1936. A method of demonstrating rust hyphae and haustoria in unsectioned leaf tissue. American Journal of Botany10:686-688.
- Rost, F. and R. Oldfield. 2000. Photography with a Microscope. United Kingdom: Cambridge University Press.
- Ruzin, Steven E. 1999. Plant microtechnique and microscopy. Vol. 198. New York: Oxford University Press
- Schedule IV Drugs, 21 C.F.R. Section 1308.14
- Shobe, W.R. and N.R. Lersten 1967. A technique for clearing and staining gymnosperm leaves. Botanical Gazette 128:150-152.
- The United States Pharmacopeia 28/The National Formulary 23; The United States Pharmacopeial Convention, Inc.: Rockville, MD, 2005.
- Upton, R., A. Graff, G. Jolliffe, R. Langerand and E. Williamson. 2011. American herbal Pharmacopoeia. Botanical pharmacognosy. Microscopic Characterization of Botanical Medicines. CRC Press. Taylor & Francis Group.