Over the last five years 3D cell cultures have begun to be readily adopted into the drug discovery pipeline due to their improved in vivo relevancy compared to traditional 2D monolayer in vitro models. However, though these models are becoming more commonly used in drug discovery studies, there is currently a major limitation in how they are analyzed as researchers are currently using methods originally developed for 2D analysis. The current paradigm for analyzing these models consists of one of the following techniques which have their own individual limitations:
- Traditional Pathology: Cell culture models can be analyzed using traditional pathology where they are removed from well plates and processed. Though this method is capable of acquiring pathological information from tissues, the size of these models makes this process super low-throughput, tedious and expensive.
- Dissolution Based Assays: Many researchers are currently using dissolution based assays where cell culture models are homogenized and analyzed using a fluorescence probe such as Promega’s CellTiter-Glo Luminescent kit. While these assays are high-throughput, they do not capture the 3D data held within these models which makes them intrinsically more valuable than traditional 2D cell culture models.
- Wide Wield Imaging: Well plates can be characterized using wide field imaging where fluorescent markers such as GFP, immunofluorescence and chemical dyes are imaged. Though these assays are high-throughput, they suffer from a fundamental limitation which is that light attenuation results in an imaging depth of only a few cell layers. Therefore, only data from the periphery of the model is depicted and there is a significant bias to only characterize the periphery of these models which differs substantially from the interior.
- Confocal Imaging: Confocal imaging of 3D cell culture models can be high-throughput through use of a high content system such as the Thermo Fisher CX7 LZR, Molecular Devices ImageXpress, Yokogawa CV7000, Perkin Elmer Opera Phenix/CLS Operata or General Electic IN CELL 6000/6500. While these devices allow for a reduction in out of plane light, improved image quality and Z-stacks, they are still limited by light attenuation and thus only the periphery of these models is characterized.
Therefore, the major limitation with the current 3D cell culture imaging paradigm is that none of the current techniques are capable of characterizing these models in their entirety and acquring truly 3D data. To address this problem, we have developed our Visikol HISTO-M reagent which rapidly renders tissues transparent. We have shown that through the application of Visikol HISTO-M reagent to 3D cell culture models that we can dramatically improve both wide field imaging and confocal microscopy.
Confocal Microscopy
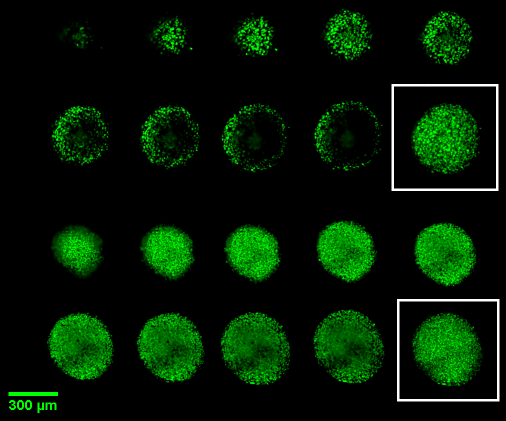
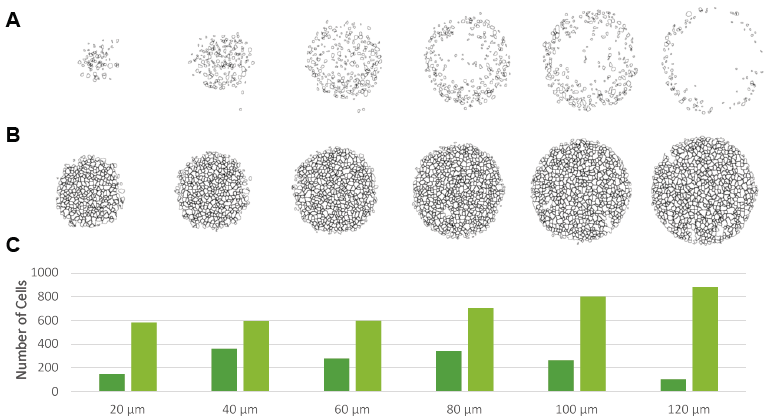
Figure 1. In this figure we show NCI-H2170 spheroids of 200 um diameter that have been cleared with Visikol HISTO-M (bottom 2 rows) and not cleared (top 2 rows). It can be seen in the optical Z projections in white boxes that because many researchers portray their data as Z projections that the current lack of complete characterization can be easily missed.
Wide Field Microscopy
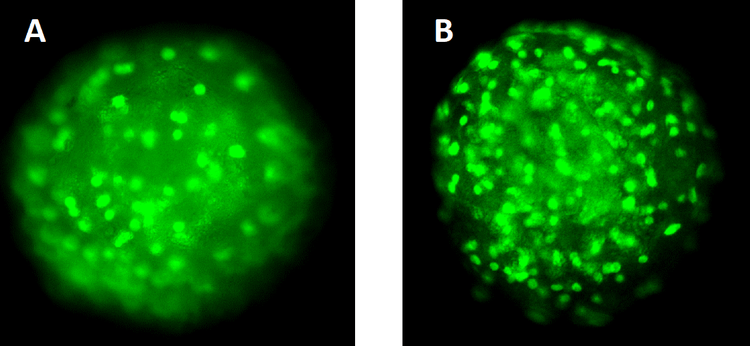
Figure 2. Visikol HISTO-M also has a substantial benefit for wide-field high content microscopy as tissue clearing allows for a 3-fold increase in cells characertized. An NCI-H2170 spheroid was labeled with SYTOX green and imaged (A) and then cleared with Visikol HISTO-M (B) and imaged. In the uncleared spheroid we were able to count 57 cells whereas we were able to count 151 cells in the cleared spheroid.
Case Study – NCI-H2170 Spheroids and Cisplatin
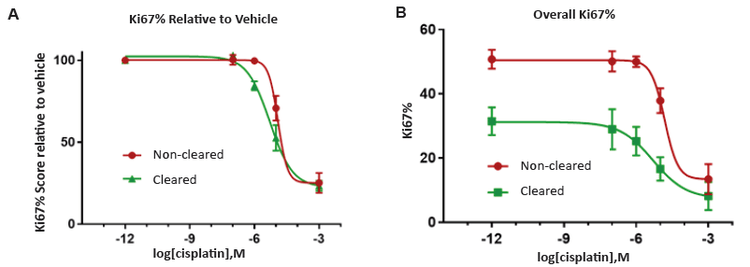
Figure 3. The application of tissue clearing was demonstrated to improve assay sensitivity as all of the cells in 3D models can be characertized instead of just the cells on the periphery.
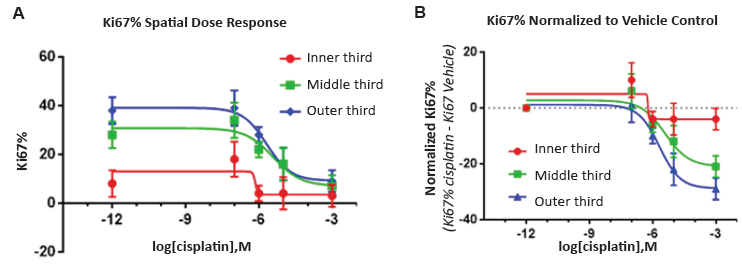
Figure 4. Visikol HISTO-M also allows for spatial dose response curves where compound and antibody penetration can be quantatively studied.
Impact
We have demonstrated that conventional assays using confocal microscopy and wide field microscopy have a tendency to bias results by only characterizing the outside of these models and that this effect is substantial. This effect can dramatically alter results for compounds or biologics that have a tendency to only target the periphery of these models as current assays could signficiantly overstate the efficacy of a compound as only the periphery is being characterized.
How can you improve your 3D cell culture assays
We currently sell our Visikol HISTO-M reagent through our online store along with the buffers and reagents required to immunolabel tissues uniformly. However, if you do not have access to a high content confocal imager or have the bandwidth to work on implementation, we run a full service lab where we can run 3D cell culture assays for clients. Check out our services page for more information.

