Non-alcoholic fatty liver disease (NAFLD) is a common condition and estimates indicate almost 1 in 4 adults in the United States have NAFLD. NAFLD occurs when fat builds up in the liver and is not tied to heavy alcohol use. There are two main types of NAFLD, non-alcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). The main difference between the two types is the presence of inflammation. The majority of people with NAFLD do not develop liver inflammation, but it’s estimated that 1.5 – 6.5% of US adults have NASH, which equates to 4 to 17 million people in the United States. Fat accumulation alone does not appear to result in significant health disparities, but the additional presence of inflammation can lead to liver damage. Scarring of the liver through deposition of collagen and fibrosis can lead to cirrhosis and liver cancer. Additional risks are also associated with NASH including Type 2 diabetes and cardiovascular disease.
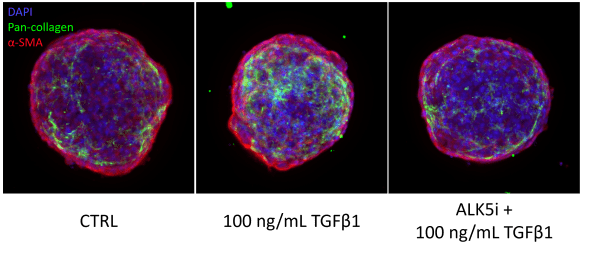
Imaging endpoints for Visikol’s 3D Human NASH/Liver Fibrosis Model, showing DAPI (blue), pan-collagen (green) and α-smooth muscle actin (red). Spheroids containing human hepatocytes and non-parenchymal cells are treated with TGFβ1 to induce fibrosis. An ALK5 inhibitor serves as a negative control, co-treatment with the ALK5 inhibitor and TGFβ1 shows a similar level of collagen deposition as the vehicle control.
Currently, there are no FDA approved treatments for NASH. As it stands, a treatment for NASH requires that a compound show decreases in inflammation with no worsening of fibrosis, decreases in fibrosis with no worsening in inflammation, or improvements in both inflammation and fibrosis in order to be considered for approval. This is also accompanied by the requirement that there is a favorable benefit-risk profile, meaning that any adverse side effects must not result in worse outcomes than the disease being treated. The drugs that have made it to clinical trials have failed due to missing one of these key endpoints. For the most part, the difficulty appears to be in decreasing both inflammation and fibrosis, with previously tested compounds typically only being effective in decreasing inflammation (e.g. Vitamin E, pioglitazone, and elafibranor).
In all, there is a real need for viable treatments for NASH, especially those that are able to show improvements in fibrosis. Early screening of potential compounds of interest can help to ascertain which may be most suited to positively impact steatosis, inflammation, and fibrosis. Visikol offers a suite of assays that can be used to study NASH, these include in vitro 2D and 3D fibrosis and steatosis assays as well as ex vivo precision cut liver slice (PCLS)-based assays. Endpoints for the assays can include quantitative PCR to look at gene expression, imaging-based endpoints to evaluate changes in α-smooth muscle actin (α-SMA) expression, collagen deposition and cell viability, and ELISA endpoints to evaluate the secretion of proteins of interest into the supernatant. These assays all have advantages in different settings, the more simplistic 2D assays can be used to screen large numbers of compounds but are more limited in the length of time the assay can be run (several days). The 3D NASH/liver fibrosis assay is slightly more complex and incorporates both hepatocytes and non-parenchymal cells in order to better recapitulate the in vivo environment. The 3D assay can be used to study longer term effects of compounds (up to several weeks). Ex vivo PCLS studies are most similar to the in vivo environment, as the slices are generated from human liver tissue directly and thus maintain the liver extra cellular matrix (ECM) structure. The slices can be cultured for up to three days with good viability and can be used to assess compound treatment more directly, but the studies are more expensive than in vitro and thus are not typically suitable for large drug screens. PCLS offers a convenient alternative or parallel to animal studies, and can be more easily related to clinical data because it uses human verses animal tissue. The entire suite of assays can be useful in different phases of the drug discovery process. If you would like to learn more about how Visikol’s NASH and steatosis models could fit into your drug discovery pipeline or if you are interested in any of the other assays Visikol offers, please reach out.
