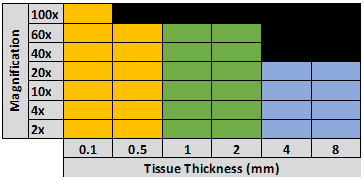
After a tissue has been labeled and rendered transparent, the last step in acquiring 3D information from your tissue is to image the tissue using a 3D imaging modality. There are three primary imaging modalities (e.g. confocal, light sheet, single/multiphoton) that can be used for this purpose and the one that you use will depend entirely upon your research question as each imaging modality has a specific set of advantages and disadvantages. More specifically, the imaging modality you chose will be based upon your desired magnification and the thickness of the tissue you are imaging. In general, confocal microscopy is best used for high resolution imaging of small volume tissues (≤ 2 mm thickness) and light sheet microscopy is best used for lower resolution imaging of large volume tissues (> 2 mm thickness).

Confocal Microscopy
Confocal microscopy is the most prevalent 3D microscopy imaging modality as it has numerous applications and allows for ultra-high-resolution imaging of tissues. However, confocal microscopy is slower than light sheet microscopy and can photo-bleach tissues as areas of tissue outside of the focal plane are illuminated during imaging. Confocal microscopy is best at high resolution imaging of small volumes where light sheet microscopy is best at low resolution imaging of large volumes. However, the specific imaging capabilities (e.g. depth, resolution) of a confocal microscope will be primarily dictated by the instruments objectives. See below for a more detailed discussion of objectives. For the depth required to image tissues thicker than 1 mm with a confocal microscope, the use of high refractive index matched, high numerical aperture immersion lenses (e.g. BABB immersion objective, glycerol immersion objective, CLARITY optimized objective, water immersion objective), or an objective with a refractive index adjusting collar is required. The maximum depth of imaging obtainable with a 10x air objectives is approximately 500-800 µm due to attenuation caused by spherical aberration. While immersion objectives can be used with inverted confocal microscopes, it is suggested that for imaging deeper than 500-800 µm that an upright confocal instrument is used. For more information on confocal microscopy, check out our Confocal Microscopy Tutorial.
Light Sheet Microscopy
The concept of light sheet microscopy has been around for almost a century and was only applied recently to the 3D visualization of tissues through the combination of tissue clearing with fluorescent labeling. Light sheet imaging can generate large volume 3D renderings of whole tissues while causing minimal photo-bleaching, but is limited in resolution compared to confocal microscopy. A light sheet microscope is very simple in nature and operates by passing an ultra-thin orthogonal laser light sheet through a cleared tissue in the same plane as the imaging objective’s focal plane. By moving the tissue up and down through the light sheet, a stack of Z projections can be generated. Most commercially available light sheet microscopes are not directly compatible with Visikol HISTO and other solvent based tissue clearing techniques. Some microscopes have a solvent compatible dipping objective and can be used without modification with Visikol HISTO and other solvent techniques. With other light sheet microscopes, you will need to mount your sample in the solvent within a double chambered cuvette, with the outer chamber filled with thiodiethanol (TDE). For more information on light sheet microscopy, check out our Light Sheet Microscopy Tutorial.
Mounting specimens
For imaging using an air objective, we recommend using the Silicone ClearWells™ included in the Visikol HISTO starter kit or available through our store. These solvent resistant silicon cut-outs stick to glass slides and are the perfect size for standard 0.15 mm coverslips. Simply stack the ClearWells until the desired depth is achieved for your tissue. Stick the ClearWells to a dry microscope slide, and press firmly to ensure a tight seal. Place the tissue in the well and fill with Visikol HISTO-2 solution. Be careful not to bump the sides of the well, as breaking the silicone seal will cause leakage.
If you are interested in learning more about this technique or any of the other many research opportunities at Visikol, please reach out to our team. We are always interested in working together with our clients as a team to develop customized assays to best suit their needs.
