 Thyroid hormones are important for regulating metabolism, development, and a variety of other body functions. While human in vitro assays are used to elucidate human-relevant interferences with thyroid gland function, they do not adequately reflect the complex TH biology and related liver-thyroid relevance; in order to address this issue, Kuhnlenz et al. developed human 3D thyroid and liver organoids, which stimulate TH biosynthesis and hepatic TH catabolism. In this study, the liver spheroids model made from HepaRG/hepatic stellate cells (HSteC) were capable of simulating TH catabolism by producing the compounds glucuronide conjugated H thyroxine (Gt4) and sulfate conjugated thyroxine 3 (St3). The ultimate goal of the study was to functionally co-cultivate pre-characterized thyroid and liver organ models on a commercially accessible multi-organ chip (MOC) technology due to the complexity of TH homeostasis, which is often impacted by both direct and indirect thyroid gland deficiencies. The co-culture setup was undertaken in order to support the functions of single-tissue organ-chip tissues for over several days.
Thyroid hormones are important for regulating metabolism, development, and a variety of other body functions. While human in vitro assays are used to elucidate human-relevant interferences with thyroid gland function, they do not adequately reflect the complex TH biology and related liver-thyroid relevance; in order to address this issue, Kuhnlenz et al. developed human 3D thyroid and liver organoids, which stimulate TH biosynthesis and hepatic TH catabolism. In this study, the liver spheroids model made from HepaRG/hepatic stellate cells (HSteC) were capable of simulating TH catabolism by producing the compounds glucuronide conjugated H thyroxine (Gt4) and sulfate conjugated thyroxine 3 (St3). The ultimate goal of the study was to functionally co-cultivate pre-characterized thyroid and liver organ models on a commercially accessible multi-organ chip (MOC) technology due to the complexity of TH homeostasis, which is often impacted by both direct and indirect thyroid gland deficiencies. The co-culture setup was undertaken in order to support the functions of single-tissue organ-chip tissues for over several days.
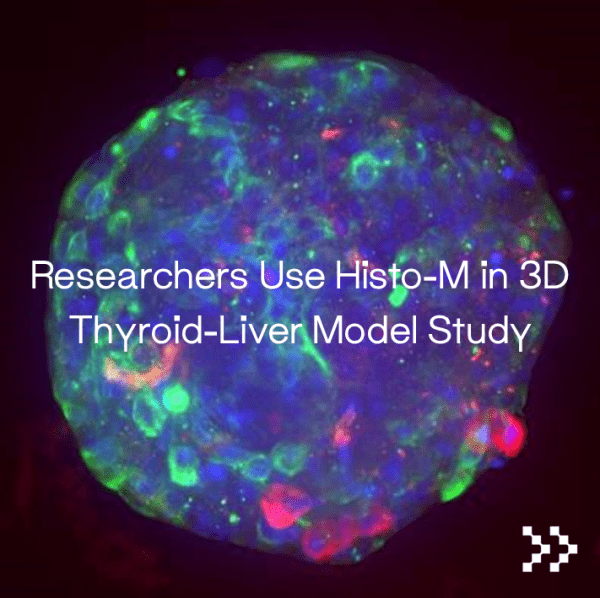
Samples were cleared in Visikol Histo-M for histological analysis, particularly for whole tissue 3D staining, which aided in the characterization of the 3D thyroid model. Thyroid model cultures exposed to different TSH concentrations showed significantly higher cAMP levels after exposure to 1 and 10 mIU/ml TSH, indicating that the 3D thyroid model is responsive to an external TSH-stimulus, authors state. Moreover, the models from four human donors were pre-cultured for three days before being treated for four days with non-cytotoxic concentrations of Methimazole (MMI), a reference Thyroid peroxidase (TPO) inhibitor. After 2 and 4 days of treatment, triiodothyronine (T3) secretion levels in culture supernatants were measured and normalized to DMSO-solvent controls; it was found that the minimal inhibitory concentration of 1 M MMI reliably inhibited T3-secretion, but after several days, it was found that 10 M MMI had the greatest inhibitory effect, suppressing T3-secretion continuously. These results suggest that the established 3D thyroid model can detect immediate TH perturbations on T3 synthesis levels. Further analysis revealed that the established liver spheroids model maintains meaningful features which contribute to an in-vivo like TH homeostasis.
Overall, the established human 3D thyroid model is the first in vitro thyroid model that can be integrated into a perfused organ-chip system with viability and TSH-dependent T3 secretion over 21 days; however, further model improvement must be considered if one wishes to follow physiological T4/T3 ratio, for instance, by understanding and observing DIO1/2 activity in the system; DIO1, the only gene expression currently under investigation, did not significantly differ from levels of expression found in primary tissues, Kuhnlenz et al. state.
Visikol offers a wide range of tissue clearing reagents for transparentizing biological tissues and cell models, as well as advanced drug discovery solutions such as 3D cell culture assays and tissue imaging high content screening, AI solutions for histological analysis of tissue sections, and much more. To learn more about Visikol’s services, please contact us.
