Accurate histological staining relies on various factors, the first crucial step being proper fixation. Tissue fixation is a technique histologists use to preserve a sample or specimen, thus preventing the natural decay process from degrading the sample. Fixation acts as a pause button on the dynamic environment within the tissue, allowing us to get a snapshot of the proteins expressed during specimen removal. Various methods are used to fix tissue samples, all of which have the same result of preserving the sample. Additionally, there are multiple ways that a specimen can be improperly fixed, leading to sample degradation and less than accurate immunolabeling results.
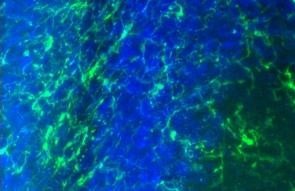
Representative images of rat brain immunolabeled with Iba1. Rat brain 3 days after mechanically induced traumatic brain injury.
Types of Fixation and Fixatives
Tissue fixation is separated into two categories: physical and chemical. Physical methods include cryo-preservation, heat fixation, and microwave fixation. Cryo-preservation is a technique in which tissue is rapidly frozen to preserve the specimen. This physical method is more common than heat fixation, which is typically used for smears of microorganisms. Despite the growing popularity of physical methods, chemical fixation methods are still the most commonly used in labs. Chemical fixation is the process in which specimens are immersed in a solution containing one or more fixative agents dissolved in a buffer. Chemical fixatives can be classified as either denaturing or cross-linking agents. Denaturing agents work by replacing the water within the tissue, changing the structure of proteins by destabilizing hydrophobic bonds. This change in the tertiary protein structure preserves the tissues for histological analysis. Cross-linking agents work by chemically reacting with proteins and other tissue components, binding them, and forming inter-molecular and intra-molecular cross-links. These cross-links preserve the tissue but also cover up important antigen-binding sites. Therefore, samples must undergo an additional antigen retrieval step prior to immunolabeling. It is important to note what type of fixation is being used on a specimen, as each impacts tissue morphology and integrity.
The Effects of Over or Under-Fixation of Specimens
Improper fixation of histological specimens can occur using any physical or chemical methods listed above. There are two main types of improper fixation of specimens: under-fixation and over-fixation. Under-fixation occurs when a specimen is not immersed in the fixative solution long enough for the fixative to permeate the tissue fully. If a tissue sample is under-fixed, it can lead to the degradation or putrefaction of the sample, rendering histologists unable to mount and stain it for imaging purposes. Over-fixation occurs when a specimen is fixed for longer than necessary, usually by leaving a specimen in a fixative solution for an extended period. An over-fixed sample can cause structural changes within the tissue, making it brittle and difficult to mount. Suppose the over-fixed sample can be mounted. In that case, histologists may still face labeling problems. Over-fixation can alter tissue morphology and antigenic mask sites on proteins, leading to inaccurate histological imaging and results.
Proper fixation of histological specimens is crucial to achieving accurate and reliable histological imaging and analysis results. To achieve optimal results, it is essential to know which fixative your lab uses, how to use it effectively, and how that fixative may impact histological analysis. Choosing the proper fixative depends on the research question, sample type, and imaging conditions. Contact our team today to help determine which fixative is best for your application.
