Example Metabolic Activity (nmoles/hr/106 cells)
| Substrate | Enzyme | Concentration (µM) | Day 1 | Day 4 | Column 8 |
|---|---|---|---|---|---|
| Midazolam | CYP3A4 | 5 | 1.153 | 1.363 | 1.077 |
| Dextromethorphan | CYP2D6 | 20 | 0.50 | .440 | .9 |
| Tolbutamide | CYP2C9 | 20 | .044 | .091 | .231 |
Culture Condition and Morphology
Pooled cryopreserved human hepatocytes were thawed and plated with HUREL PlatinumHeps™ Media supplemented with 10% serum and subsequently changed 24 hours post-seeding to HUREL PlatinumHeps™ Basal Media. CYP substrate concentrations are given in the table above along with metabolite formation recorded as nmoles/hr/106 cells. All incubations were carried out in triplicate on days 1, 4, and 8 upon cell delivery and incubated for 60 minutes. Reactions took place in a humidified incubator at 37°C, in 5% CO2. Collected supernatants were stored at -20°C until further LC/MS/MS analysis.
Day 1 – Morphology
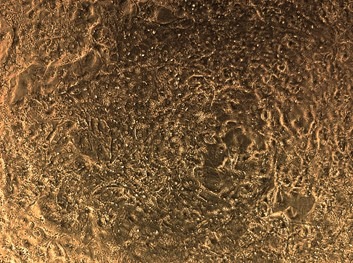
Phase contrast image in a 24-well at a 10x magnification.
Day 7 – Morphology
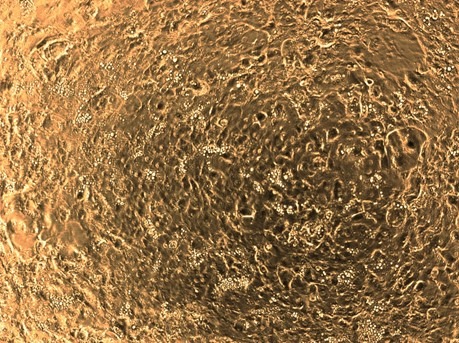
Phase contrast image in a 24-well at a 10x magnification.
Day 7 – Bile Canaliculi
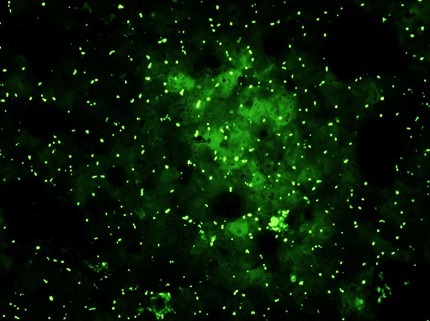
Bile canaliculi assayed via 5-(and-6)-carboxy-2’, 7’-dichlorofluorescein diacetate (C-DCFDA) stain at a concentration of 5 µM and imaged in the GFP channel in a 96-well at 10x magnification with filters EX/EM 492-495/512-527 nm.
Example Culture Origin
Human Donor Demographics
- Gender: Male(2) Female(3)
- Age (yrs)” 40-70
- Race: Cauc.(4) African American(1)
- Cause of Death: Anoxia(1), N/A(4)
- Tobacco History: Not (2) Past Use (3)
- Alcohol History: Past Use(2) Current Use (3)
- Medical History: Not Given.
Donor Serology
- Hepatitis B: Negative
- Hepatitis C: Negative
- HIV: Negative
- EBV: IgG [+](5), IgG [-}(1), N/A (4)
- RPR: Negative
- CMV: Positive (6) Negative (4)
