Most researchers struggle with the antibody labeling associated with tissue clearing and 3D tissue imaging. What researchers typically experience is that they are unable to achieve uniform labeling with their antibody across the depth of their tissue. To address this problem we have outlined the protocol described below:
Obtain and Fix tissue of interest
The general process for fixing tissues is to fix tissues in 4% PFA overnight at 4°C followed by one hour of clearing at room temperature. The tissue is then placed in PBS with azide (0.04% w/v).
It is best to use a 24-well plate or eppendorf tubes to contain samples for treatment steps for small sections. However trim tissue to whatever containers are available, Eppendorf tubes are an excellent choice as well.
Tissue Preparation and Antigen Retrieval
1. Cut 100-250 µm sections of the tissue using a vibratome. Cut 5 sections for each antibody intended for validation and optimization.
2. Incubate tissues in PBS for 5 minutes.
3. Remove PBS with pipet, add 1 mL methanol, incubate tissues for 15 minutes.
4. (Optional step for highly pigmented tissues e.g. non-perfused tissues, kidney, liver.) Prepare (using refrigerated reagents stored at 4°C) 5% H2O2 in 20% DMSO/methanol by adding 1 mL 30% H2O2 (careful! Strong oxidizer!) to 4 mL methanol. Add 1 mL DMSO. This solution should be kept at 4°C until use. Incubate tissue in 1 mL of this solution at 4°C for 30 minutes.
5. Remove solution from tissue and add 1 mL of 20% DMSO/80% methanol, incubate for 15 minutes.
6. Incubate tissues in 1 mL 100% methanol for 15 minutes.
7. Incubate tissues in 1 mL PBS with 1% Triton X-100 for 15 minutes.
Immunolabeling
8. Incubate tissues in ~400 µL (increase volume as necessary to completely cover tissue) penetration buffer for 15 minutes.
9. Incubate tissue in ~400 µL (increase volume as necessary to completely cover tissue) blocking buffer for 15 minutes.
10. Incubate tissue in ~400 µL (increase volume as necessary to completely cover tissue) antibody buffer containing varying dilutions of antibody ranging from 1:50 to 1:500 (e.g. 1:50, 1:100, 1:200, 1:300, 1:500. Incubate for 30 minutes.
11. Wash tissues by exchanging buffer with washing buffer 5x with 5 minutes incubation time between exchanges.
12. (If using indirect labeling with secondary antibody) Incubate tissue in ~400 µL (increase volume as necessary to completely cover tissue) antibody buffer containing 1:200 dilution of secondary antibody (concentration may also need optimization using this method of varying dilution, depending on distribution of fluorophore).
13. (Optional step if using indirect labeling with secondary antibody) Wash tissues by exchanging buffer with washing buffer 5x with 5 minutes incubation time between exchanges.
Clearing
14. Transfer to 1 mL 100% methanol for 30 minutes.
15. Transfer tissues to 400-500 µL Visikol HISTO-1 for 10-30 minutes.
16. Remove from polystyrene well plate and transfer to slide. Mount in 400-500 µL Visikol HISTO-2.
Imaging
Validation of staining of antibodies can be accomplished simply by using a typical fluorescent microscope. Prepare a slide of the cleared tissue and quickly examine for specificity of signal.
To examine evenness of staining, image tissues using a confocal microscope. Obtain a z-stack spanning the entire thickness of the tissue section with two color channels: the channel corresponding to the fluorescent conjugate for antibody staining, and the channel used for nuclear stain. Since nuclear stains penetrate tissues rapidly and homogenously, the nuclear stain channel serves as a control for optical attenuation.
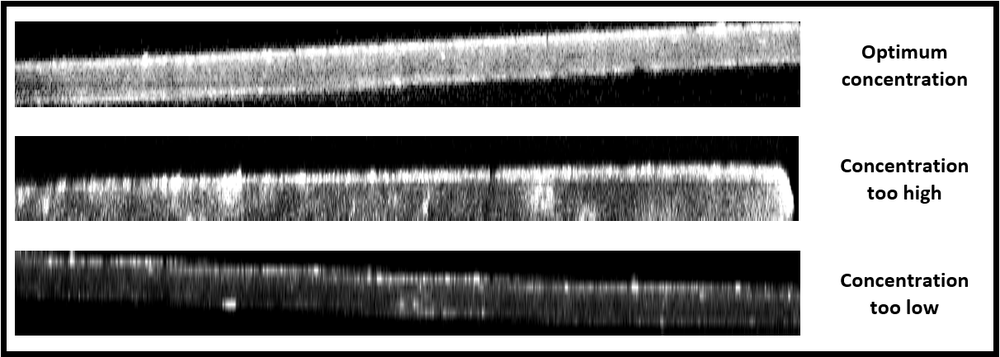
Examine the z-stacks in ImageJ (or other image processing software). Observe the XZ and YZ plane by viewing “Orthogonal Views” to examine for evenness of staining. If staining is even, you should see relatively constant intensity (with respect to nuclear stain) across the tissue (see table below). Some dimming in inner layers is expected, but signal should be visible across tissue.
If the concentration of the immunolabel is too high, you will see a bright ring of staining at the surface layers, with uneven staining at a lower intensity into the tissue (see table below). If the concentration of the immunolabel is too low, you will see slight staining at the surface layer, with a dark interior and uneven spots of stain.
Visualization of staining with XZ planes of image stacks of tissue sections