Background
- Hepatic cholestasis involves the abnormal accumulation of bile acids in bile canaliculi (BC) and is implicated as a common mechanism of drug-induced liver injury.
- Determination of potential toxic liabilities for drug compounds requires evaluation of cholestatic potential of drug candidates.
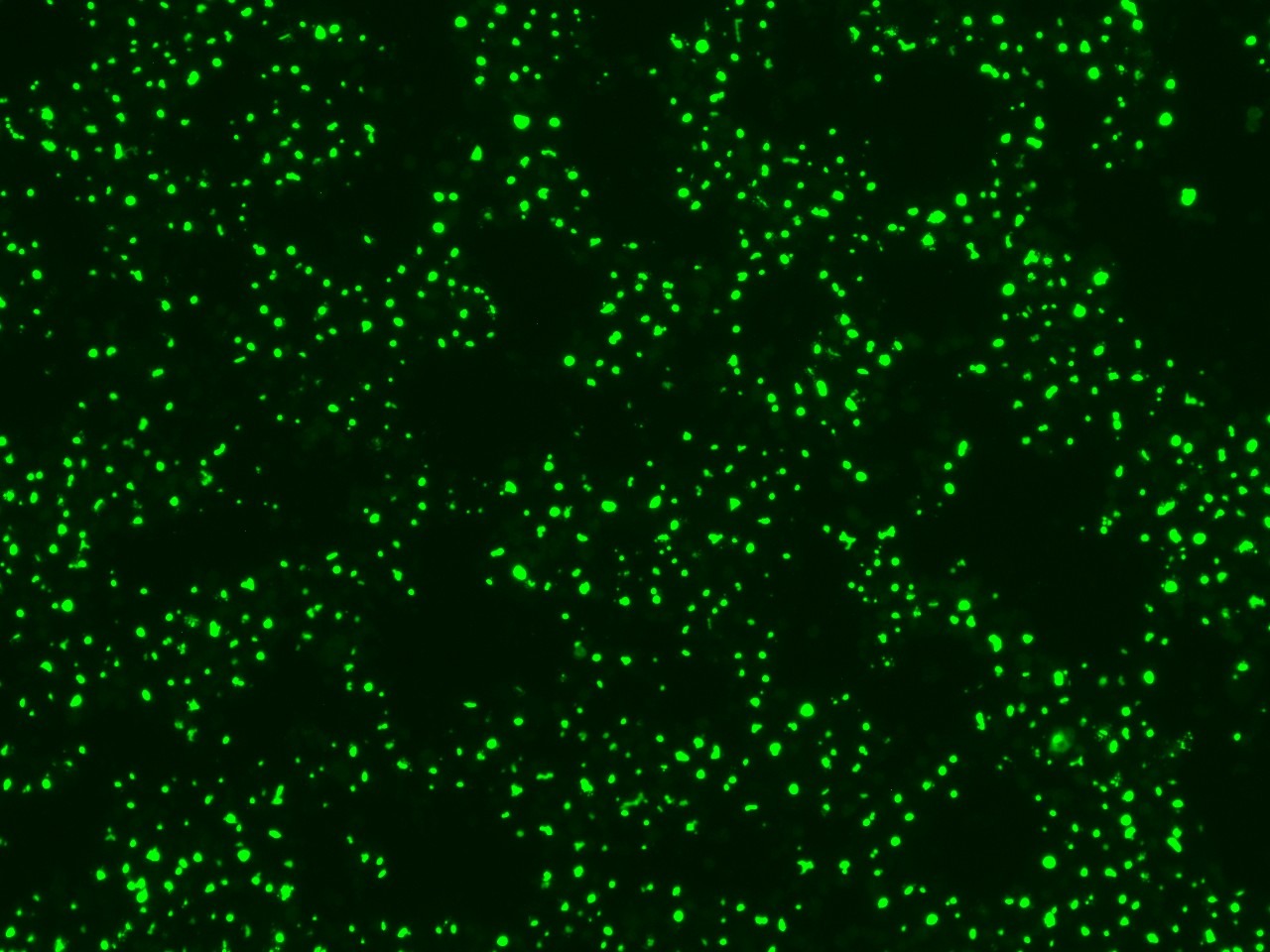
- Utilizing fluorescent bile acid probes, direct measurement of bile salt accumulation can be conducted using high content imaging.
- While it has long been suggested that inhibition of bile salt export pumps (BSEP) underlies abnormal bile acid accumulation, more recent studies have found that known BSEP-inhibiting compounds are not associated with liver injury or that many cholestatic drugs are not BSEP inhibitors. Therefore, simple evaluation of BSEP inhibition is often insufficient in the evaluation of a compound’s cholestatic liability.
- Alternatively, bile canalicular dynamics have been suggested to provide a more comprehensive evaluation of cholestatic liabilities.
- This assay, which utilizes fluorescent bile acid probes and direct measurement of bile salt accumulation via high content imaging, enables the evaluation of BC dilation and constriction in the context of compound-induced cholestasis.
Protocol
| Instrument | Molecular Devices ImageExpress Micro Confocal |
| Analysis Method | High content screening |
| Markers | Fluorescent bile acid probe |
| Cell Types Available | HepaRG, HepG2 Primary human hepatocytes, Primary Cynomolgus monkey hepatocytes, primary canine hepatocytes |
| Cell model | 2D cell monolayer |
| Test Article Concentration | 8 point assay (0.1, 0.5, 1, 5, 10, 50, 100, 500 µM) (custom concentrations available) |
| Number of Replicates | 3 replicates per concentration |
| Quality Controls | 0.5% DMSO (vehicle control) Chlorpromazine (positive control) |
| Test Article Requirements | 50 uL of 20 mM solution or equivalent amount of solid |
| Data Delivery | Dose response curves, EC50 values, mean bile canaliculi area for each dose point |
General Procedure
- Cells grown to 80% confluent monolayer
- Treatment with test compounds
- Addition of fluroescent bile acid probe
- Fixation of cells
- Cells counterstained with DAPI
- High content imaging is conducted on well plates
- Images are analyzed to quantify bile acid salt accumulation and size of bile canaliculi
Figure 1. Representative image depicting fluorescent bile salt probe accumulation in bile canaliculi in HepaRG cells
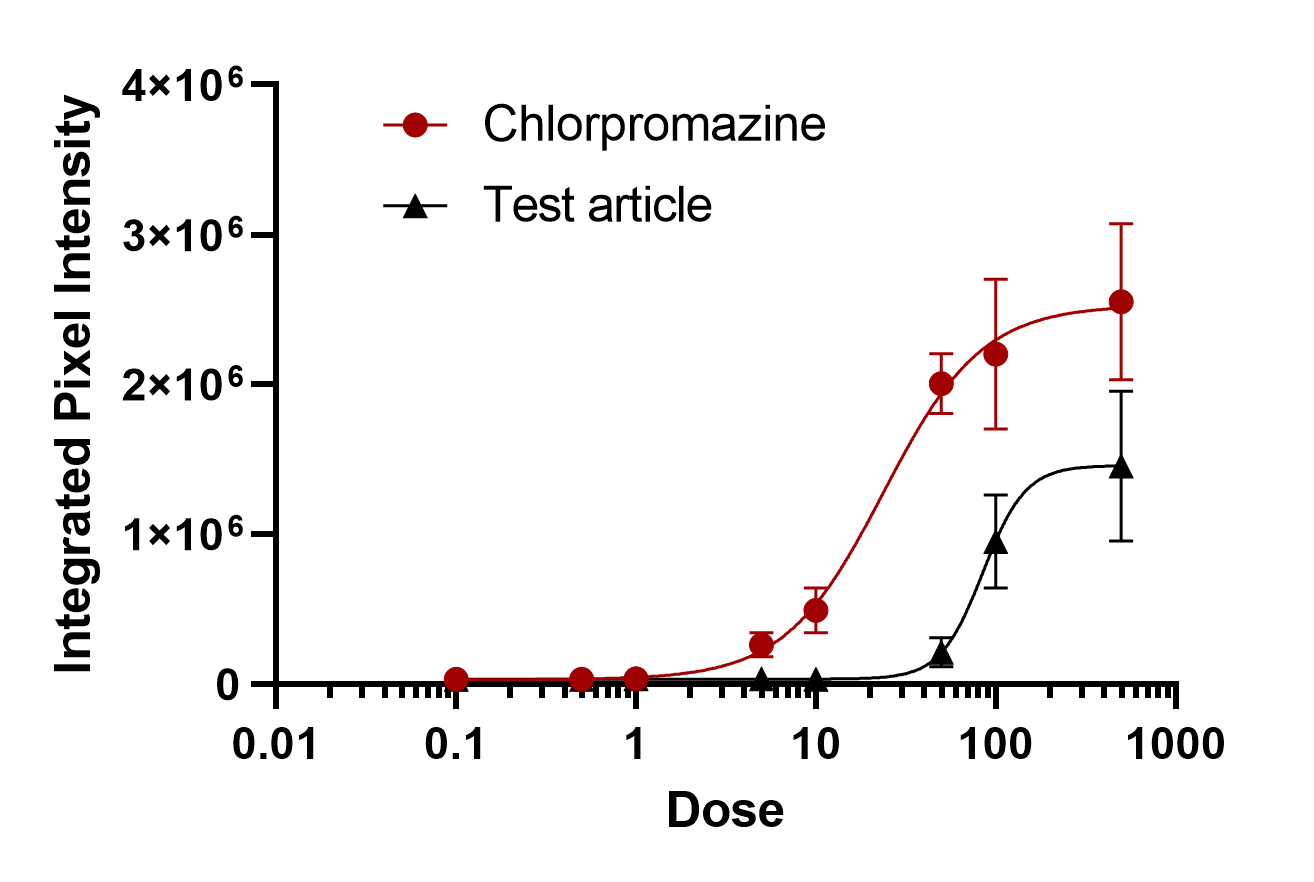
Figure 2. Dose response curve depicting test article (EC50 85.03 uM) and positive control chlorpromazine (EC50 23.82 uM)
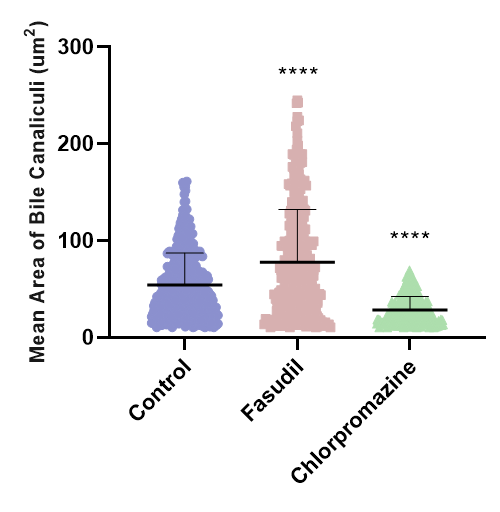
Figure 3. Distribution of mean bile canaliculi area for control cells, fasudil-treated and chlorpromazine-treated HepaRG cells. Fasudil is known to promote dilation of bile canaliculi, while chlorpromazine is known to constrict bile canaliculi.
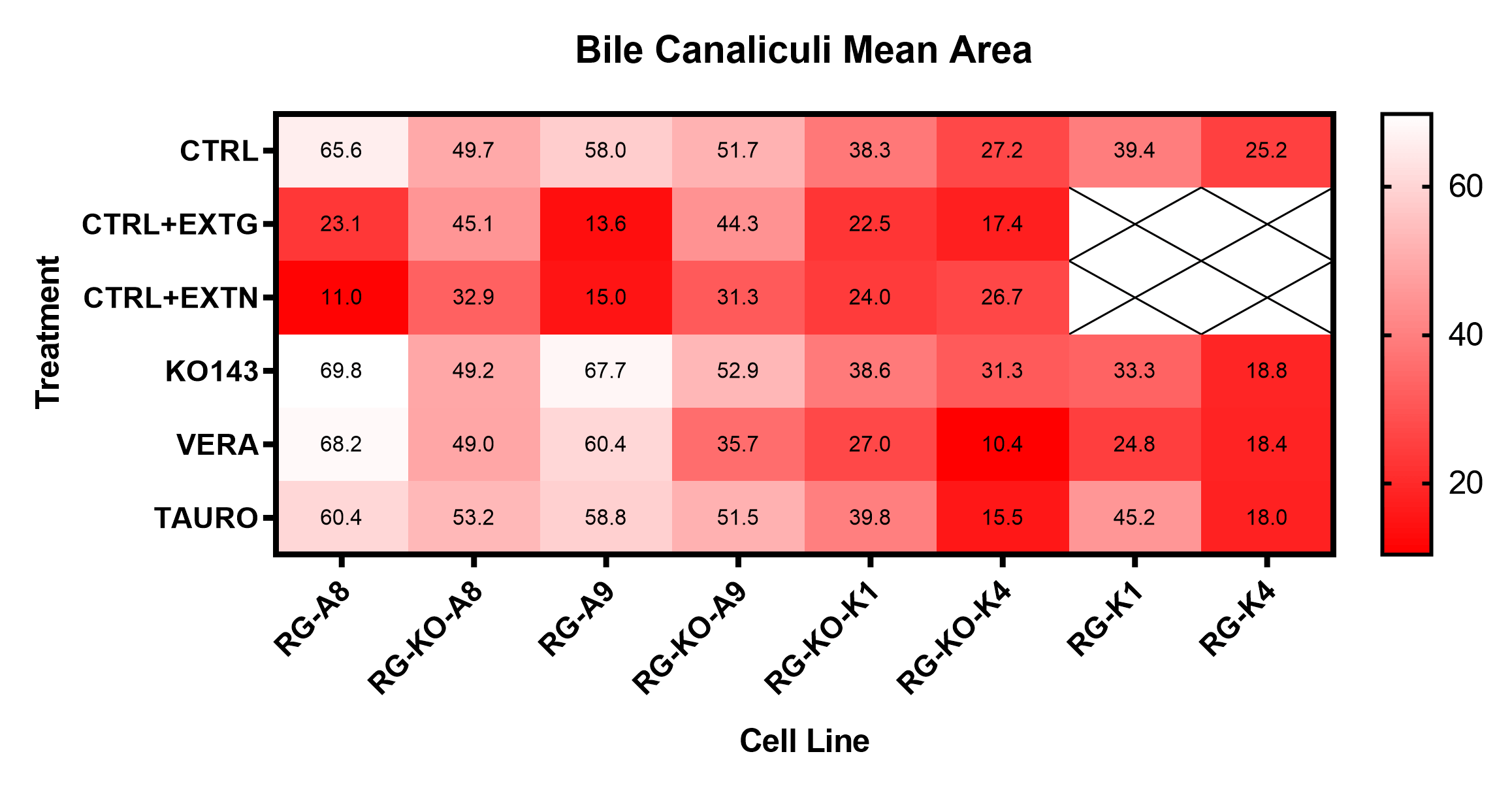
Figure 4. Representative heatmap depicting mean bile canaliculi area for various genetically modified HepaRG cells with various treatments