Characterizing the transport properties of biologicals (e.g. large molecules, antibody-based therapeutics, etc.) in tissues is a complex task that, until recently, has required costly, time consuming in vivo studies. Generally speaking, large molecules are distributed throughout tissues via convective transport. While in healthy tissues, extracellular fluid flows steadily from capillaries to lymphatic vessels, driving transport of macromolecules throughout the tissue, transport of large molecules through more complex tissues (i.e. diseased tissues, solid tumors) can be encumbered by several transport-limiting factors. For example, in the tumor microenvironment, lymphatic vessels may be dysfunctional, altering hydrostatic pressure, and consequently interrupting the convection gradient responsible for driving large molecule transport through the local tissue microenvironment [1, 2]. Once permeated into a tissue, diffusion, convection, and availability of and affinity to target antigens- factors which may be also affected by alterations to the tissue microenvironment- are responsible for the balance of large molecule distribution.
As the interest in using biologicals for therapeutic advantage has grown, so has interest in evaluating the ways that antibody derivatives, antibody drug conjugates, antibody fragments (e.g. Fab), alternate domain antibodies (e.g. camelids), affibodies, or nanobodies may differently penetrate target tissues of interest. While studies comparing the pharmacokinetics of these various biologicals have typically relied upon costly, time consuming in vivo studies, researchers are increasingly turning to in vitro models to offer earlier, faster, more-cost effective insight prior to embarking on these later in vivo studies.
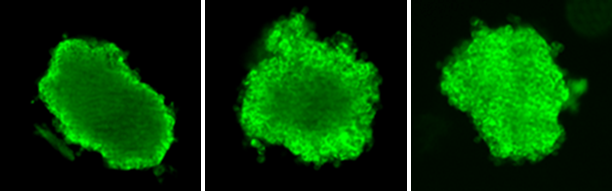
Accordingly, at Visikol, we have developed a method in which the distribution of these large molecules and their derivatives within 3D cell culture models (i.e. spheroids) or excised whole tissues or tissue pieces can be evaluated in vitro. Briefly, this protocol involves generation of an appropriate 3D cell culture model, dosing of that model with a test article at varying doses and durations, and then fixing the model for subsequent labeling, including fluorescent detection of the test article. Several defining characteristics of therapeutic penetration, including depth and quantity of penetration can then be analyzed. To put this in context, internal investigations using an IgG targeted to integrin β1 in HepG2 tumor spheroids revealed that penetration depth is affected primarily by duration of therapeutic antibody incubation, but the total amount of antibody penetrated is dependent on both the dose and duration of antibody incubation (data can be found on our antibody pharmacokinetics assay page).
Moreover, this assay can be multiplexed with cytotoxicity or colocalization assays (i.e. with cell subtype markers or a target identifying marker) to provide further insight into the cellular distribution and functional implications of therapeutic penetration. Contact Visikol today to learn how you can leverage this assay in your research studies (info@visikol.com).
[1] Thurber, G. M., Schmidt, M. M., & Wittrup, K. D. (2008). Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Advanced drug delivery reviews, 60(12), 1421-1434.
[2] Ryman, J. T., & Meibohm, B. (2017). Pharmacokinetics of monoclonal antibodies. CPT: pharmacometrics & systems pharmacology, 6(9), 576-588.