In this recent blog post from Dr. Ramesh Dasari and Dr. Amir Wahba from Visikol, they evaluate the ability to cryopreserve mouse kidney slices. As a company, Visikol is highly focused on providing clients with advanced cell culture assays and one of the many models that Visikol offers its clients are tissue slice assays. While these assays leverage highly relevant in vivo tissues in an in vitro format, the availability of tissues makes the assays operationally challenging to execute, and thus having a cryopreservation approach allows for easy scheduling and execution. Within this work, Dr. Dasari and Dr. Wahba look at multiple functional parameters of kidneys before and after cryopreservation to evaluate the approach for routine use.
Cryopreservation has become a key enabling technology in regenerative and translational medicine to provide a stable and secure extended cell storage for primary tissue isolates and cell preparations. Cryopreservation can bring about a number of cellular injuries, potentially leading to deleterious changes in cell morphology, characteristics, metabolic activity, function, and cell death. Temperature decrease can be responsible for triggering specific stress response pathways and can activate apoptotic and necrotic pathways after thawing. However, with appropriate precautions adopted during the methodology, cryopreservation would overcome the shortage of human tissues during emergency conditions.
Precision cut tissue slices (PCTS) represent a suitable and convenient tool for pharmacological, toxicological and morphological studies. We investigated the potential of cryopreservation of mouse kidney slices as a morphological tool by snap freezing the slices (without any medium) in liquid nitrogen and freezing them at -80oC. The application of a rapid freezing method (direct immersion in liquid nitrogen) allows for maintaining urea synthesis, sulfo-conjugation, and CYP-dependent oxidation of ethoxycoumarin, testosterone hydroxylation, and N-de-ethylation of lidocaine at the same level as a non-cryopreserved rat or human PCLS after 2 or 3 hours of incubation.
Mouse kidney tissues were procured from Melior Discovery. The tissues were packaged in a temperature-controlled container and maintained on ice until transported to Visikol. Processing began immediately by obtaining 5 mm biopsy punch cores, followed by cutting using the Compresstome automatic tissue slicers. The cores were bonded to sample holders one at a time, embedded in agarose, mounted, and sectioned (section thickness: 250μm). The slices were collected in a Petri dish containing ice-cold UW solution and immediately transferred to cryovials which were snap-frozen in liquid nitrogen and stored at -80oC. Cryopreserved kidney slices were thawed by transferring the freeze vials directly from liquid nitrogen into a water bath at 37oC. After thawing, slices were washed with slice washing medium (DPBS supplemented with 1x B27 minus insulin, trypsin inhibitor, aprotinin, chymostatin and D-glucose) at room temperature. Then the slices were cultured in 24-well transwell inserts up to 72 hours in culture medium ( William’s E medium supplemented with 1x B27 minus insulin, trypsin inhibitor, aprotinin, chymostatin, 1% FBS, 1x insulin transferrin-selenium, dexamethasone, GlutaMAX and D-glucose). Before the end of the incubation period, the slices were cultured with substrates (midazolam, tolbutamide, dextromethorphan, and phenacetin) for one hour and the conditioned medium was collected. The CYP metabolites were separated on an Agilent PoroShell 120 EC-C18 column with an isocratic solvent system composed of 90% H2O (0.1% (v/v) acetic acid) and 10% ACN (0.1% (v/v) acetic acid) (Figure 1).
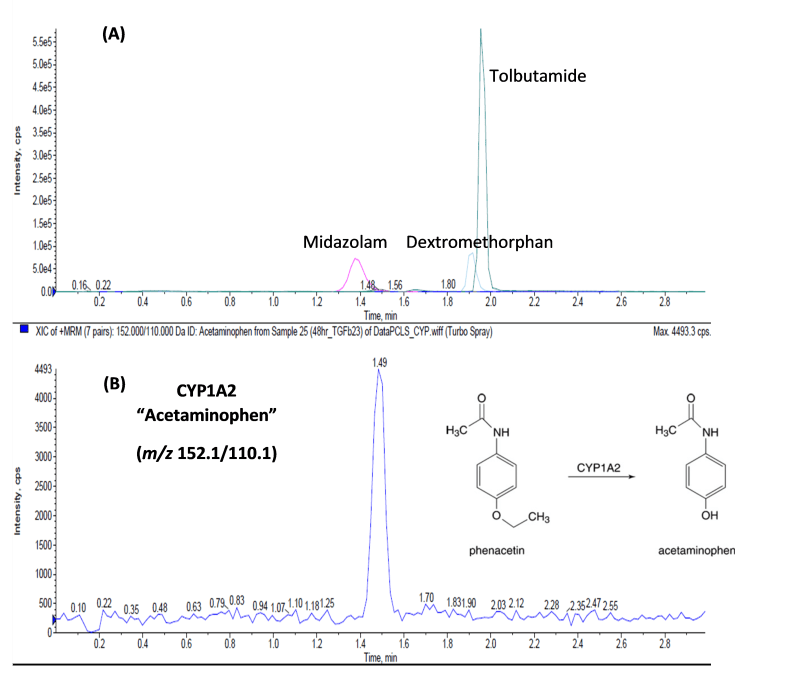
Figure 1. (A) LC-MS/MS chromatogram of the conditioned medium of kidney PCTS following one-hour incubation with CYP enzyme substrates: midazolam, tolbutamide, dextromethorphan, and phenacetin. (B) Selected base peak of acetaminophen, the metabolic product of CYP1A2, at m/z 152.1/110.1, which was the only enzyme that showed activity. The metabolites were separated on an Agilent PoroShell 120 EC-C18 column with an isocratic solvent system composed of 90% H2O (0.1% (v/v) acetic acid) and 10% ACN (0.1% (v/v) acetic acid).
Our results indicate that phase I metabolic enzymes were well maintained after cryopreservation in kidney slices. While this does not guarantee that this cryopreservation approach will maintain all functionality within the slices, it does demonstrate a viable path towards preserving tissue slices for routine use over long periods of time from the same donor.
Therefore, if you are looking to adopt PCTS assays into your work please reach out to Visikol, we have a lot of flexibility in designing assays and can work to develop a solution that best meets your research needs.
