 The first time I learned this figure I frankly did not believe it (check it out for yourself). I asked, “How could this be when there are so many companies focused on fluorescent multiplexing and so many proprietary and open source (i.e., multiplexing via bleaching) approaches to conducting multiplexing?” Surely, most researchers would actually assume that the opposite was in fact true. After looking at the publications for myself, I realized that this was in fact true despite how many fluorescent approaches there are to multiplexing (including ours at Visikol). But the question remains of how this is possible as it seems incongruous to what we are seeing in the marketplace with so many researchers interested in fluorescent multiplexing which appears to have a many-fold larger population of followers.
The first time I learned this figure I frankly did not believe it (check it out for yourself). I asked, “How could this be when there are so many companies focused on fluorescent multiplexing and so many proprietary and open source (i.e., multiplexing via bleaching) approaches to conducting multiplexing?” Surely, most researchers would actually assume that the opposite was in fact true. After looking at the publications for myself, I realized that this was in fact true despite how many fluorescent approaches there are to multiplexing (including ours at Visikol). But the question remains of how this is possible as it seems incongruous to what we are seeing in the marketplace with so many researchers interested in fluorescent multiplexing which appears to have a many-fold larger population of followers.
Now, I don’t know for sure but can speculate why this is so. Simply put, fluorescent multiplexing and multiplex imaging in general are very challenging for several reasons:
Antibody Validation for Multiplex Tissue Imaging:
For anyone who has tried to validate a new antibody with a new tissue, they know it is challenging and determining true signal from background noise is a qualitative process that can be very challenging for low expression epitopes. When you multiply this complexity by 20 and have to take into account the sequence of labeling, the interplay between different fluorophores, the changing nature of the tissue and epitopes through multiple rounds of processing and you have a very complicated validation problem which gets exponentially more challenging the more markers you use. It is for this reason that fluorescent multiplexing is simply just very technically challenging beyond 10 or so markers. Further, an antibody validation effort for 20+ markers is simply just expensive from a materials and labor perspective as each primary antibody typically has an average cost of $400 per vial plus shipping.
Processing Data from Multiplex Imaging:
For every channel that you are imaging on a full slide with fluorescent multiplexing you are generating approximately 2GB of data. When you multiply that by 20 channels and let’s say 20 slides, you have a tremendous amount of data. Computationally registering these channels to each other and conducting subsequent analysis is also a major bottle neck which many researchers simply don’t have the bandwidth, expertise or hardware for.
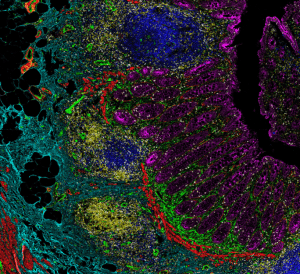
Why Many Researchers Turn Towards IMC:
Based on these challenges, most researchers like our Visikol lab seldom go above 10 IHC markers on a single slide which is why you will see that as the number of markers goes down, the proportion of publications using fluorescence based multiplexing dramatically increases. Intuitively, the proportion of publications using multiplex imaging is directly proportional to the exponential increase in complexity associated with additional markers (i.e., more markers = less publications with fluorescence. IMC is truly a special tool for high plex IHC imaging as it has a few very unique features:
- Single Step Labeling: All of the labeling is conducted in a single step. With IMC, primary antibodies are conjugated to metals which allows for all of these antibodies to be used for labeling in a single step. This removes the problem of sequence where the sequence of antibody labeling needs to be validated with fluorescent multiplexing in addition to the antibodies themselves.
- Low Data Density: More data is not always better and in life science imaging increasing data means reduced throughput, higher cost and increased complexity. IMC provides a unique balance of a high number of labels with a moderate resolution (1 um pixels) and data files that are able to be easily processed.
- Low Signal to Noise: One of the most unique aspects of IMC is that because non-naturally occurring metals are conjugated to antibodies, there is a very low degree of background noise compared to fluorescent imaging. These means that there is a high degree of sensitivity and even low expressing epitopes can be easily imaged.
I see the fluorescent multiplexing approach as quite analogous to the tissue clearing and 3D imaging space where you can image a whole mouse brain for example at 40X in 3D for multiple antibodies but you probably shouldn’t. Each tool has a unique niche in which it is most efficacious and it appears based on the publication data that as you approach 20 markers, a researcher should shift their focus from fluorescence to IMC for the considerations outlined above whereas both have their unique use cases in which they are most effective. For more background on multiplex tissue imaging check out the resources below:

Author
Michael Johnson, Ph.D.
MatTek Life Sciences CCO/Visikol General Manager
