Immunohistochemistry (IHC) and immunofluorescence (IF) are standard molecular imaging techniques for labeling specific proteins within tissues. For both methods, ensuring the accessibility of antigens of interest for antibody binding is crucial for accurate and reliable results. Two of the most common methods for antigen retrieval are pressure cooking and boiling, each with distinct advantages and disadvantages.
Pressure-Cooker Method
The pressure-cooking method of antigen retrieval entails immersing fixed tissue slides in an antigen retrieval solution and subjecting them to high pressure and temperature for a short period, typically utilizing either an electronic or microwavable pressure cooker. This process breaks the covalent bonds formed during fixation, rendering the antigens more accessible for antibody binding. This technique offers efficiency and uniformity, with retrieval typically completed in 15-30 minutes. It also maintains tissue morphology better than many other alternative methods. However, its dependence on specialized equipment and the potential risk of tissue damage may limit its applicability. It is essential to make sure that the tissue slides do not dry out during the retrieval process, which can cause severe tissue damage. However, the use of a closed pressure cooker makes it more challenging to monitor the slides while they are undergoing retrieval. Commonly used antigen retrieval solutions for the pressure-cooking method include citrate buffer (pH 6.0) and EDTA buffer (pH 9.0). Still, the type of buffer used may vary depending on tissue type, tissue integrity, target proteins of interest, and antibodies used for labeling and imaging.
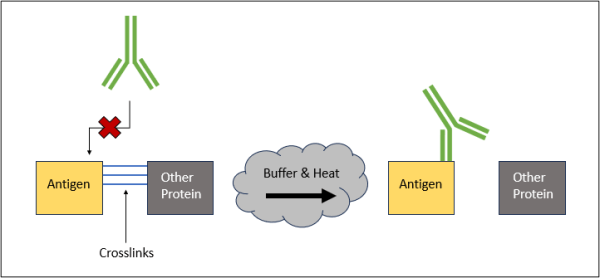
Graphical representation of the cross-linking reaction that takes place following antigen retrieval. When tissue samples are fixed, cross-linking occurs to preserve the tissue. For antibodies to bind to their target proteins, we must remove the cross-links and make the antibody binding sites accessible.
Boiling Technique
In contrast, the boiling technique involves immersing tissue slides in an antigen retrieval solution and heating them in a boiling water bath. Like the pressure-cooking method, this method utilizes heat to open the binding sites by disrupting formalin-induced cross-links. However, not as much pressure is created when using this method, leading to longer retrieval times. Boiling is a more accessible and cost-effective method, particularly for labs with limited resources. It is versatile and suitable for various tissue types and antigens. However, retrieval efficiency can vary significantly, potentially leading to non-specific staining and increased autofluorescence if the slides are boiled for too long. The process may also be time-consuming, taking up to an hour or more, depending on the protocol, tissue type, and buffers utilized. Commonly used antigen retrieval solutions for the boiling method include citrate buffer (pH 6.0) and Tris-EDTA buffer (pH 9.0).

An example of a set up for utilizing the boiling antigen retrieval method.

An example of a microwavable pressure cooker that can be utilized for the pressure cooker antigen retrieval.
Beyond pressure cooking and boiling, other antigen retrieval techniques exist:
-Enzymatic digestion involves treating tissue sections with enzymes like trypsin or pepsin to break down proteins and enhance antigen accessibility. This method is beneficial for formalin-fixed paraffin-embedded tissues.
-Microwave heating is similar to the boiling method but with a controlled microwave setting. This method can be effective for antigen retrieval, though careful optimization is required to avoid tissue damage.
-The ultrasonic bath technique uses ultrasound waves to facilitate antigen retrieval by disrupting formalin-induced cross-links. This method suits delicate tissues that would not hold up under high heat or pressure.
-Chemical treatments can also be used for antigen retrieval, often using chemical agents like urea or guanidine hydrochloride to disrupt cross-links and enhance antigen accessibility. This method requires careful optimization of concentration and incubation time.
Choosing the Right Antigen Retrieval Method
Ultimately, the choice of antigen retrieval method depends on factors such as tissue type, antigen specificity, and available resources. Researchers should weigh the advantages and disadvantages of each technique to select the most suitable approach for their specific experimental needs. Regardless of the chosen method, rigorous optimization and validation are essential for achieving consistent and accurate results in immunohistochemistry and immunofluorescence studies. chosen method, rigorous optimization and validation are essential for achieving consistent and accurate results in immunohistochemistry and immunofluorescence studies.
If you’re interested in learning more, reach out to a member of our team today!
