 Lung cancer has predominated as one of the leading causes of death worldwide, with lung cancer patients facing a poor prognosis more often than not. The general inefficacy of lung cancer treatments is due to various factors, including disease heterogeneity, late diagnosis, and aggressiveness of lung cancers. The traditional approaches, chemotherapies and radiation, are often off-target and can inflict unwanted side effects, thereby increasing systemic cytotoxicity. To try and combat this, Immunotherapies have emerged as a popular approach given that they utilize our body’s built-in immune system to combat cancers. However, immunotherapies for lung cancer treatment exhibit poor success rates primarily due to the lack of understanding of the molecular and cellular drivers behind immune changes in response to tumors across the diversity of cancer stages and grades.
Lung cancer has predominated as one of the leading causes of death worldwide, with lung cancer patients facing a poor prognosis more often than not. The general inefficacy of lung cancer treatments is due to various factors, including disease heterogeneity, late diagnosis, and aggressiveness of lung cancers. The traditional approaches, chemotherapies and radiation, are often off-target and can inflict unwanted side effects, thereby increasing systemic cytotoxicity. To try and combat this, Immunotherapies have emerged as a popular approach given that they utilize our body’s built-in immune system to combat cancers. However, immunotherapies for lung cancer treatment exhibit poor success rates primarily due to the lack of understanding of the molecular and cellular drivers behind immune changes in response to tumors across the diversity of cancer stages and grades.
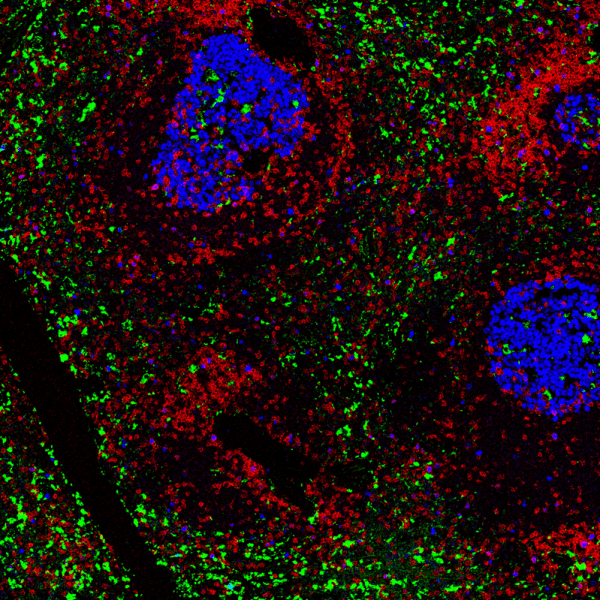
The current model to classify tumors is based on a tumor-node-metastasis (TNM) classification system. The limitation to this system is that patients can have the same TNM but vastly different treatment responses. This is because the model does not account for crucial biochemical, metabolic, and genetic features that are unique to each patient tumor. Immunoscoring aims to bridge this gap by measuring immune cell infiltration as a predictor for how a patient will respond to immunotherapies. In a recent study entitled “Spatially variant immune infiltration scoring in human cancer tissues,” researchers from the Georgia Institute of Technology and Emory University investigated the tumor immune architecture at a single-cell level to quantify the heterogeneity of lung cancers using highly multiplexed IMC. An H&E stained tumor microarray (12 tissues corresponding to 6 lung cancer patients) was pre-annotated by a pathologist to identify the regions of interest (ROIs), with those ROIs being areas that exhibited varying cancer stages, grades, and immune infiltration patterns. IMC was used to target 26 markers that identified stromal, immune, and cancer cell states: CD8α, CD68, CD163, CD206, HLA-DR, PD-1, PD-L1, granzyme B, FoxP3, CD20, CD4, CD3, CD45RO, TCF1, CD103, and CD95 for immune markers; pan-keratin and e-cadherin for cancerous/paracancerous regions; and SMA and COL1 for stromal regions.
Single-cell analysis was performed using segmentation via a deep-learning technique and CellProfiler, in conjunction with unsupervised clustering. Further, the spatial relationship among cell types was quantified using cell neighborhood analysis to generate cell proximity maps that were overlaid on the tissue images from IMC. These spatial maps were then used to generate infiltration maps that indicated a continuum distribution across samples ranging from immune cold tumors (very little immune cell infiltration), to immune suppressed tumors (immune microenvironment was inhibited), and immune hot tumors (inflamed immune microenvironments).
Overall, this study shows the wide spectrum and diversity of cell types in tumor tissues, which helps to explain the differential in patient responses to immune-based treatments. The methodology used demonstrates the value that IMC can add to a study, as well as the potential for IMC to provide deeper insight into experimental results. Visikol is proud to announce the addition of IMC to our repertoire of services, and we are confident that our advanced imaging capabilities can help our Clients answer even their most complex research questions. Reach out today to find out how Visikol can help accelerate your research.
