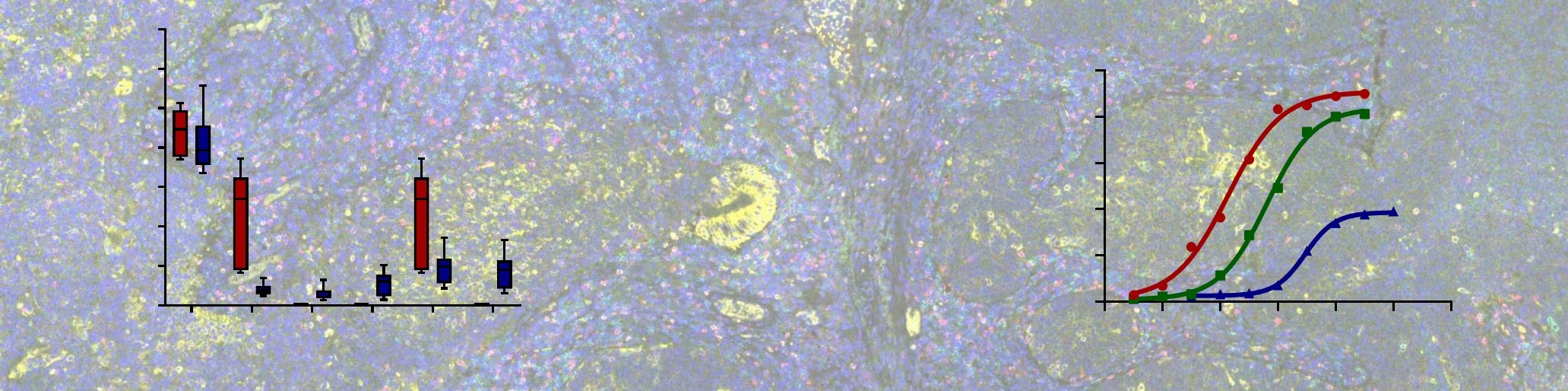
Steatosis and Phospholipidosis Assay
Rapidly assess compounds in vitro for hepatotoxicity
Hepatotoxicity and Drug Compounds
Hepatotoxicity is one of the leading causes of attrition in drug development pipelines. Steatosis and phospholipidosis are disorders of the liver which cause abnormal retention of triglycerides and phospholipids, respectively. These conditions can be induced by hepatotoxic drug compounds, and can lead to delays in the drug development process if not properly assessed and mitigated. In vitro assessment of drug-induced steatosis and phospholipidosis is an important part of the screening process.
With Visikol, you can pursue the cutting edge of in vitro models, bioimaging, and tissue analysis to exploit the wealth of information contained within every experiment. The unique approach we take to conducting drug discovery research allows our clients to get more out of their in vitro screening campaigns, giving more accurate and more reliable information for making better informed decisions early in the pipeline.
More relevant
3D cell culture models better mimic the in vivo microenvironment and better mimic response to drug treatment, giving you more reliable data earlier in the discovery process.
See more
Using high content confocal imaging, assay multiple endpoints simultaneously, giving you more data about your compounds faster.
Take action
Our expert team processes images and analyzes data, delivering actionable insights in an easy-to-read report, so you can make decisions and push forward your drug discovery efforts.